Psychiatric Manifestation of Anti-LGI1 Encephalitis
- PMID: 32560097
- PMCID: PMC7348933
- DOI: 10.3390/brainsci10060375
Psychiatric Manifestation of Anti-LGI1 Encephalitis
Abstract
Background: Anti-leucine-rich glioma-inactivated 1 (LGI1) encephalitis is typically characterized by limbic encephalitis, faciobrachial dystonic seizures and hyponatremia. The frequency with which milder forms of anti-LGI1 encephalitis mimic isolated psychiatric syndromes, such as psychoses, or may lead to dementia if untreated, is largely unknown.
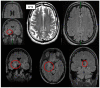
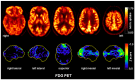
Case presentation: Here, the authors present a 50-year-old patient who had suffered from neurocognitive deficits and predominant delusions for over one and a half years. He reported a pronounced feeling of thirst, although he was drinking 10-20 liters of water each day, and he was absolutely convinced that he would die of thirst. Due to insomnia in the last five years, the patient took Z-drugs; later, he also abused alcohol. Two years prior to admission, he developed a status epilepticus which had been interpreted as a withdrawal seizure. In his serum, anti-LGI1 antibodies were repeatedly detected by different independent laboratories. Cerebrospinal fluid analyses revealed slightly increased white blood cell counts and evidence for blood-brain-barrier dysfunction. Magnetic resonance imaging showed hyperintensities mesio-temporally and in the right amygdala. In addition, there was a slight grey-white matter blurring. A cerebral [18F] fluorodeoxyglucose positron emission tomography (FDG-PET) examination of his brain showed moderate hypometabolism of the bilateral rostral mesial to medial frontal cortices. Treatment attempts with various psychotropic drugs remained unsuccessful in terms of symptom relief. After the diagnosis of probable chronified anti-LGI1 encephalitis was made, two glucocorticoid pulse treatments were performed, which led to a slight improvement of mood and neurocognitive deficits. Further therapy was not desired by the patient and his legally authorized parents.
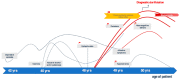
Conclusion: This case study describes a patient with anti-LGI1 encephalitis in the chronified stage and a predominant long-lasting psychiatric course with atypical symptoms of psychosis and typical neurocognitive deficits. The patient's poor response to anti-inflammatory drugs was probably due to the delayed start of treatment. This delay in diagnosis and treatment may also have led to the FDG-PET findings, which were compatible with frontotemporal dementia ("state of damage"). In similar future cases, newly occurring epileptic seizures associated with psychiatric symptoms should trigger investigations for possible autoimmune encephalitis, even in patients with addiction or other pre-existing psychiatric conditions. This should in turn result in rapid organic clarification and-in positive cases-to anti-inflammatory treatment. Early treatment of anti-LGI1 encephalitis during the "inflammatory activity state" is crucial for overall prognosis and may avoid the development of dementia in some cases. Based on this case, the authors advocate the concept-long established in many chronic inflammatory diseases in rheumatology-of distinguishing between an "acute inflammatory state" and a "state of organ damage" in autoimmune psychosis resembling neurodegenerative mechanisms.
Keywords: anti-LGI1 encephalitis; autoimmune psychosis; limbic encephalitis.
Conflict of interest statement
D.E.: None. H.P.: None. A.D.: None. J.S.: None. B.F.: None. T.S.: None. N.V.: None. K.N.: None. S.M.: None. M.M.: None. S.J.M.: None. K.D.: Steering Committee Neurosciences, Janssen. H.U.: He is a shareholder of Veobrain GmbH. P.T.M.: None. L.T.v.E.: Advisory boards, lectures, or travel grants within the last three years: Roche, Eli Lilly, Janssen-Cilag, Novartis, Shire, UCB, GSK, Servier, Janssen and Cyberonics.
Figures
Similar articles
-
Anti-leucine-rich glioma-inactivated 1 encephalitis revealed by a manic episode: insights from frontal lobe dysfunction in neuropsychiatry through neuropsychology and metabolic imaging. A case report.Front Psychiatry. 2023 May 18;14:1168302. doi: 10.3389/fpsyt.2023.1168302. eCollection 2023. Front Psychiatry. 2023. PMID: 37275973 Free PMC article.
-
Antibody-LGI 1 autoimmune encephalitis manifesting as rapidly progressive dementia and hyponatremia: a case report and literature review.BMC Neurol. 2019 Feb 7;19(1):19. doi: 10.1186/s12883-019-1251-4. BMC Neurol. 2019. PMID: 30732585 Free PMC article. Review.
-
Case Report: Anti-LGI1 Limbic Encephalitis Associated With Anti-thyroid Autoantibodies.Front Neurol. 2021 Jan 15;11:620483. doi: 10.3389/fneur.2020.620483. eCollection 2020. Front Neurol. 2021. PMID: 33519701 Free PMC article.
-
Anti-LGI1 encephalitis preceded by psychiatric symptoms: A case report.PCN Rep. 2024 Mar 6;3(1):e181. doi: 10.1002/pcn5.181. eCollection 2024 Mar. PCN Rep. 2024. PMID: 38868479 Free PMC article.
-
Clinical characteristics of Leucine-rich glioma-inactivated protein 1 antibody-mediated autoimmune encephalitis in a 6-year-old girl: case report and literature reviews.BMC Neurol. 2023 Jun 30;23(1):253. doi: 10.1186/s12883-023-03299-z. BMC Neurol. 2023. PMID: 37391712 Free PMC article. Review.
Cited by
-
Anti-leucine-rich glioma-inactivated 1 encephalitis revealed by a manic episode: insights from frontal lobe dysfunction in neuropsychiatry through neuropsychology and metabolic imaging. A case report.Front Psychiatry. 2023 May 18;14:1168302. doi: 10.3389/fpsyt.2023.1168302. eCollection 2023. Front Psychiatry. 2023. PMID: 37275973 Free PMC article.
-
Neuropsychological Performance in Autoimmune Limbic Encephalitis: Evidence from an Immunotherapy-Naïve Cohort.Arch Clin Neuropsychol. 2022 May 16;37(4):738-752. doi: 10.1093/arclin/acac001. Arch Clin Neuropsychol. 2022. PMID: 35136904 Free PMC article.
-
Leucine-rich Glioma Inactivated 1 (LGI-1) Limbic Encephalitis Presenting with Psychotic Symptoms without Seizures: A Case Report with Five-year Follow-up and Review of Literature.Indian J Psychol Med. 2024 Jul;46(4):367-370. doi: 10.1177/02537176231226191. Epub 2024 Feb 11. Indian J Psychol Med. 2024. PMID: 39056044 Free PMC article. No abstract available.
-
Anti-LGI1-antibody autoimmune encephalitis as a neurological paraneoplastic syndrome associated with gastrointestinal stromal tumour: a case report.Postep Psychiatr Neurol. 2025 Jun;34(2):108-115. doi: 10.5114/ppn.2025.150016. Epub 2025 Jun 23. Postep Psychiatr Neurol. 2025. PMID: 40666614 Free PMC article.
-
Leveraging molecular biomarkers to make the common diagnosis in the uncommon patient.J Neuroimmunol. 2021 Mar 15;352:577474. doi: 10.1016/j.jneuroim.2021.577474. Epub 2021 Jan 7. J Neuroimmunol. 2021. PMID: 33461093 Free PMC article.
References
-
- Van Sonderen A., Thijs R.D., Coenders E.C., Jiskoot L.C., Sanchez E., de Bruijn M.A., van Coevorden-Hameete M.H., Wirtz P.W., Schreurs M.W., Sillevis Smitt P.A., et al. Anti-LGI1 encephalitis: Clinical syndrome and long-term follow-up. Neurology. 2016;87:1449–1456. doi: 10.1212/WNL.0000000000003173. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

