Future Climate Significantly Alters Fungal Plant Pathogen Dynamics during the Early Phase of Wheat Litter Decomposition
- PMID: 32560135
- PMCID: PMC7356542
- DOI: 10.3390/microorganisms8060908
Future Climate Significantly Alters Fungal Plant Pathogen Dynamics during the Early Phase of Wheat Litter Decomposition
Abstract
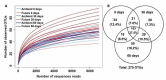
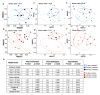
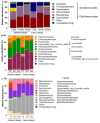
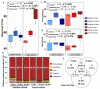
Returning wheat residues to the soil is a common practice in modern agricultural systems and is considered to be a sustainable practice. However, the negative contribution of these residues in the form of "residue-borne pathogens" is recognized. Here, we aimed to investigate the structure and ecological functions of fungal communities colonizing wheat residues during the early phase of decomposition in a conventional farming system. The experiment was conducted under both ambient conditions and a future climate scenario expected in 50-70 years from now. Using MiSeq Illumina sequencing of the fungal internal transcribed spacer 2 (ITS2), we found that plant pathogenic fungi dominated (~87% of the total sequences) within the wheat residue mycobiome. Destructive wheat fungal pathogens such as Fusarium graminearum, Fusarium tricinctum, and Zymoseptoria tritci were detected under ambient and future climates. Moreover, future climate enhanced the appearance of new plant pathogenic fungi in the plant residues. Our results based on the bromodeoxyuridine (BrdU) immunocapture technique demonstrated that almost all detected pathogens are active at the early stage of decomposition under both climate scenarios. In addition, future climate significantly changed both the richness patterns and the community dynamics of the total, plant pathogenic and saprotrophic fungi in wheat residues as compared with the current ambient climate. We conclude that the return of wheat residues can increase the pathogen load, and therefore have negative consequences for wheat production in the future.
Keywords: GCEF; MiSeq Illumina sequencing; climate change; fungal ITS2; litter decomposition; mycobiome; wheat straw decomposition.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Klocke N.L., Currie R.S., Aiken R.M. Soil water evaporation and crop residues. Trans. ASABE. 2009;52:103–110. doi: 10.13031/2013.25951. - DOI
LinkOut - more resources
Full Text Sources

