Quinoxaline Derivatives as Antiviral Agents: A Systematic Review
- PMID: 32560203
- PMCID: PMC7356203
- DOI: 10.3390/molecules25122784
Quinoxaline Derivatives as Antiviral Agents: A Systematic Review
Abstract
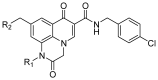
Background: In recent decades, several viruses have jumped from animals to humans, triggering sizable outbreaks. The current unprecedent outbreak SARS-COV-2 is prompting a search for new cost-effective therapies to combat this deadly pathogen. Suitably functionalized polysubstituted quinoxalines show very interesting biological properties (antiviral, anticancer, and antileishmanial), ensuring them a bright future in medicinal chemistry.
Objectives: Focusing on the promising development of new quinoxaline derivatives as antiviral drugs, this review forms part of our program on the anti-infectious activity of quinoxaline derivatives.
Methods: Study compiles and discusses recently published studies concerning the therapeutic potential of the antiviral activity of quinoxaline derivatives, covering the literature between 2010 and 2020.
Results: A final total of 20 studies included in this review.
Conclusions: This review points to a growing interest in the development of compounds bearing a quinoxaline moiety for antiviral treatment. This promising moiety with different molecular targets warrants further investigation, which may well yield even more encouraging results regarding this scaffold.
Keywords: SAR; antiviral; biological applications; chemistry; quinoxaline.
Conflict of interest statement
The authors declare no conflict of interest.
Figures














References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

