The Emerging Role and Promise of Circular RNAs in Obesity and Related Metabolic Disorders
- PMID: 32560220
- PMCID: PMC7349386
- DOI: 10.3390/cells9061473
The Emerging Role and Promise of Circular RNAs in Obesity and Related Metabolic Disorders
Abstract
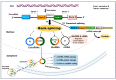
Circular RNAs (circRNAs) are genome transcripts that are produced from back-splicing of specific regions of pre-mRNA. These single-stranded RNA molecules are widely expressed across diverse phyla and many of them are stable and evolutionary conserved between species. Growing evidence suggests that many circRNAs function as master regulators of gene expression by influencing both transcription and translation processes. Mechanistically, circRNAs are predicted to act as endogenous microRNA (miRNA) sponges, interact with functional RNA-binding proteins (RBPs), and associate with elements of the transcriptional machinery in the nucleus. Evidence is mounting that dysregulation of circRNAs is closely related to the occurrence of a range of diseases including cancer and metabolic diseases. Indeed, there are several reports implicating circRNAs in cardiovascular diseases (CVD), diabetes, hypertension, and atherosclerosis. However, there is very little research addressing the potential role of these RNA transcripts in the occurrence and development of obesity. Emerging data from in vitro and in vivo studies suggest that circRNAs are novel players in adipogenesis, white adipose browning, obesity, obesity-induced inflammation, and insulin resistance. This study explores the current state of knowledge on circRNAs regulating molecular processes associated with adipogenesis and obesity, highlights some of the challenges encountered while studying circRNAs and suggests some perspectives for future research directions in this exciting field of study.
Keywords: adipogenesis; adipose tissue browning; cardiovascular diseases; circular RNAs (circRNAs); epigenetics; insulin resistance; microRNAs (miRNAs).
Conflict of interest statement
The author declares no conflict of interest.
Figures
References
-
- Diederichs S., Bartsch L., Berkmann J.C., Fröse K., Heitmann J., Hoppe C., Iggena D., Jazmati D., Karschnia P., Linsenmeier M., et al. The dark matter of the cancer genome: aberrations in regulatory elements, untranslated regions, splice sites, non-coding RNA and synonymous mutations. EMBO Mol. Med. 2016;8:442–457. doi: 10.15252/emmm.201506055. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

