Sex-Specific Differences in Primary CNS Lymphoma
- PMID: 32560244
- PMCID: PMC7352658
- DOI: 10.3390/cancers12061593
Sex-Specific Differences in Primary CNS Lymphoma
Abstract
Sex-specific differences have been increasingly recognized in many human diseases including brain cancer, namely glioblastoma. Primary CNS lymphoma (PCNSL) is an exceedingly rare type of brain cancer that tends to have a higher incidence and worse outcomes in male patients. Yet, relatively little is known about the reasons that contribute to these observed sex-specific differences. Using a population-representative cohort of patients with PCNSL with dense magnetic resonance (MR) imaging and digital pathology annotation (n = 74), we performed sex-specific cluster and survival analyses to explore possible associations. We found three prognostically relevant clusters for females and two for males, characterized by differences in (i) patient demographics, (ii) tumor-associated immune response, and (iii) MR imaging phenotypes. Upon a multivariable analysis, an enhanced FoxP3+ lymphocyte-driven immune response was associated with a shorter overall survival particularly in female patients (HR 1.65, p = 0.035), while an increased extent of contrast enhancement emerged as an adverse predictor of outcomes in male patients (HR 1.05, p < 0.01). In conclusion, we found divergent prognostic constellations between female and male patients with PCNSL that suggest differential roles of tumor-associated immune response and MR imaging phenotypes. Our results further underline the importance of continued sex-specific analyses in the field of brain cancer.
Keywords: DLBCL; PCNSL; microenvironment; multimodal data; sex-specific analyses.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
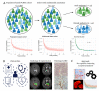

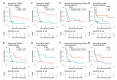
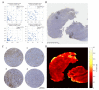
Similar articles
-
Primary central nervous system lymphoma and atypical glioblastoma: multiparametric differentiation by using diffusion-, perfusion-, and susceptibility-weighted MR imaging.Radiology. 2014 Sep;272(3):843-50. doi: 10.1148/radiol.14132740. Epub 2014 May 3. Radiology. 2014. PMID: 24814181
-
Radiomics features to distinguish glioblastoma from primary central nervous system lymphoma on multi-parametric MRI.Neuroradiology. 2018 Dec;60(12):1297-1305. doi: 10.1007/s00234-018-2091-4. Epub 2018 Sep 19. Neuroradiology. 2018. PMID: 30232517
-
Primary central nervous system diffuse large B-cell lymphoma has poorer immune cell infiltration and prognosis than its peripheral counterpart.Histopathology. 2015 Nov;67(5):625-35. doi: 10.1111/his.12706. Epub 2015 May 19. Histopathology. 2015. PMID: 25829022
-
Systems biology of primary CNS lymphoma: from genetic aberrations to modeling in mice.Acta Neuropathol. 2014 Feb;127(2):175-88. doi: 10.1007/s00401-013-1202-x. Epub 2013 Nov 16. Acta Neuropathol. 2014. PMID: 24240734 Review.
-
MRI as a diagnostic biomarker for differentiating primary central nervous system lymphoma from glioblastoma: A systematic review and meta-analysis.J Magn Reson Imaging. 2019 Aug;50(2):560-572. doi: 10.1002/jmri.26602. Epub 2019 Jan 14. J Magn Reson Imaging. 2019. PMID: 30637843
Cited by
-
Radiomic features define risk and are linked to DNA methylation attributes in primary CNS lymphoma.Neurooncol Adv. 2023 Oct 18;5(1):vdad136. doi: 10.1093/noajnl/vdad136. eCollection 2023 Jan-Dec. Neurooncol Adv. 2023. PMID: 38024240 Free PMC article.
-
Impact of thiotepa dose-intensity in primary diffuse large B-cell lymphoma of the central nervous system undergoing autologous hematopoietic cell transplant with thiotepa/carmustine conditioning.Bone Marrow Transplant. 2023 Nov;58(11):1203-1208. doi: 10.1038/s41409-023-02071-8. Epub 2023 Aug 10. Bone Marrow Transplant. 2023. PMID: 37563283 Free PMC article.
-
Non-cancer-specific survival in patients with primary central nervous system lymphoma: A multi-center cohort study.Front Oncol. 2023 Feb 8;13:1096027. doi: 10.3389/fonc.2023.1096027. eCollection 2023. Front Oncol. 2023. PMID: 36845683 Free PMC article.
References
-
- Caetano M.S., Hassane M., Van H.T., Bugarin E., Cumpian A.M., McDowell C.L., Cavazos C.G., Zhang H., Deng S., Diao L., et al. Sex specific function of epithelial STAT3 signaling in pathogenesis of K-ras mutant lung cancer. Nat. Commun. 2018;9:4589. doi: 10.1038/s41467-018-07042-y. - DOI - PMC - PubMed
-
- Yang W., Warrington N.M., Taylor S.J., Whitmire P., Carrasco E., Singleton K.W., Wu N., Lathia J.D., Berens M.E., Kim A.H., et al. Sex differences in GBM revealed by analysis of patient imaging, transcriptome, and survival data. Sci. Transl. Med. 2019;11:eaao5253. doi: 10.1126/scitranslmed.aao5253. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

