Various Anti-HSPA2 Antibodies Yield Different Results in Studies on Cancer-Related Functions of Heat Shock Protein A2
- PMID: 32560263
- PMCID: PMC7352356
- DOI: 10.3390/ijms21124296
Various Anti-HSPA2 Antibodies Yield Different Results in Studies on Cancer-Related Functions of Heat Shock Protein A2
Abstract
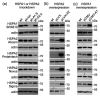
Heat shock proteins (HSPs) constitute a major part of the molecular chaperone system and play a fundamental role in cell proteostasis. The HSPA (HSP70) family groups twelve highly homologous HSPA proteins. Certain HSPAs are regarded as important cancer-related proteins, prospective therapeutic targets for cancer treatment, and also as potential cancer biomarkers. Heat Shock Protein A2 (HSPA2), a testis-enriched chaperone and one of the least characterized members of the HSPA family, has recently emerged as an important cancer-relevant protein with potential biomarker significance. Nevertheless, conflicting conclusions have been recently drawn both according to HSPA2 role in cancer cells, as well as to its prognostic value. In this work we have shown that one of the serious limitations in HSPA2 protein research is cross-reactivity of antibodies marketed as specific for HSPA2 with one or more other HSPA(s). Among non-specific antibodies were also those recently used for HSPA2 detection in functional and biomarker studies. We showed how using non-specific antibodies can generate misleading conclusions on HSPA2 expression in non-stressed cancer cells and tumors, as well as in cancer cells exposed to proteotoxic stress. Our findings addressed concerns on some published studies dealing with HSPA2 as a cancer-related protein.
Keywords: HSPA2; antibody specificity; cancer biomarker; heat shock protein family A; immunohistochemistry; proteotoxic stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Heat shock protein A2 is a novel extracellular vesicle-associated protein.Sci Rep. 2023 Mar 23;13(1):4734. doi: 10.1038/s41598-023-31962-5. Sci Rep. 2023. PMID: 36959387 Free PMC article.
-
Functional redundancy of HSPA1, HSPA2 and other HSPA proteins in non-small cell lung carcinoma (NSCLC); an implication for NSCLC treatment.Sci Rep. 2019 Oct 7;9(1):14394. doi: 10.1038/s41598-019-50840-7. Sci Rep. 2019. PMID: 31591429 Free PMC article.
-
Expression, function, and regulation of the testis-enriched heat shock HSPA2 gene in rodents and humans.Cell Stress Chaperones. 2015 Mar;20(2):221-35. doi: 10.1007/s12192-014-0548-x. Epub 2014 Oct 25. Cell Stress Chaperones. 2015. PMID: 25344376 Free PMC article. Review.
-
Inhibition of the Heat Shock Protein A (HSPA) Family Potentiates the Anticancer Effects of Manumycin A.Cells. 2021 Jun 7;10(6):1418. doi: 10.3390/cells10061418. Cells. 2021. PMID: 34200371 Free PMC article.
-
Heat Shock Protein A2 (HSPA2): Regulatory Roles in Germ Cell Development and Sperm Function.Adv Anat Embryol Cell Biol. 2017;222:67-93. doi: 10.1007/978-3-319-51409-3_4. Adv Anat Embryol Cell Biol. 2017. PMID: 28389751 Review.
Cited by
-
Heat shock protein A2 is a novel extracellular vesicle-associated protein.Sci Rep. 2023 Mar 23;13(1):4734. doi: 10.1038/s41598-023-31962-5. Sci Rep. 2023. PMID: 36959387 Free PMC article.
-
Effect of Curcumin on Hepatic mRNA and lncRNA Co-Expression in Heat-Stressed Laying Hens.Int J Mol Sci. 2024 May 15;25(10):5393. doi: 10.3390/ijms25105393. Int J Mol Sci. 2024. PMID: 38791430 Free PMC article.
-
HSPA2 Chaperone Contributes to the Maintenance of Epithelial Phenotype of Human Bronchial Epithelial Cells but Has Non-Essential Role in Supporting Malignant Features of Non-Small Cell Lung Carcinoma, MCF7, and HeLa Cancer Cells.Cancers (Basel). 2020 Sep 24;12(10):2749. doi: 10.3390/cancers12102749. Cancers (Basel). 2020. PMID: 32987811 Free PMC article.
-
Numerical modelling and experimental verification of thermal effects in living cells exposed to high-power pulses of THz radiation.Sci Rep. 2021 Sep 9;11(1):17916. doi: 10.1038/s41598-021-96898-0. Sci Rep. 2021. PMID: 34504144 Free PMC article.
-
HSPA2 influences the differentiation and production of immunomodulatory mediators in human immortalized epidermal keratinocyte lines.Cell Death Dis. 2025 Apr 26;16(1):344. doi: 10.1038/s41419-025-07565-5. Cell Death Dis. 2025. PMID: 40287440 Free PMC article.
References
-
- Boudesco C., Cause S., Jego G., Garrido C. Hsp70: A Cancer Target Inside and Outside the Cell. Methods Mol. Biol. 2018;1709:371–396. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

