Inhibition of Transglutaminase 2 but Not of MDM2 Has a Significant Therapeutic Effect on Renal Cell Carcinoma
- PMID: 32560270
- PMCID: PMC7349864
- DOI: 10.3390/cells9061475
Inhibition of Transglutaminase 2 but Not of MDM2 Has a Significant Therapeutic Effect on Renal Cell Carcinoma
Abstract
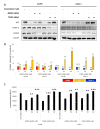
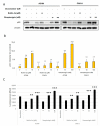
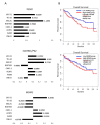
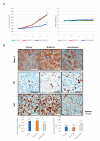
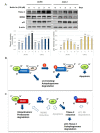
More than 50% of human cancers harbor TP53 mutations and increased expression of Mouse double minute 2 homolog(MDM2), which contribute to cancer progression and drug resistance. Renal cell carcinoma (RCC) has an unusually high incidence of wild-type p53, with a mutation rate of less than 4%. MDM2 is master regulator of apoptosis in cancer cells, which is triggered through proteasomal degradation of wild-type p53. Recently, we found that p53 protein levels in RCC are regulated by autophagic degradation. Transglutaminase 2 (TGase 2) was responsible for p53 degradation through this pathway. Knocking down TGase 2 increased p53-mediated apoptosis in RCC. Therefore, we asked whether depleting p53 from RCC cells occurs via MDM2-mediated proteasomal degradation or via TGase 2-mediated autophagic degradation. In vitro gene knockdown experiments revealed that stability of p53 in RCC was inversely related to levels of both MDM2 and TGase 2 protein. Therefore, we examined the therapeutic efficacy of inhibitors of TGase 2 and MDM2 in an in vivo model of RCC. The results showed that inhibiting TGase 2 but not MDM2 had efficient anticancer effects.
Keywords: MDM2; nutlin-3a; p53; transglutaminase 2.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Iwakuma T., Lozano G. MDM2, an introduction. Mol. Cancer Res. 2003;1:993–1000. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

