Pathological Findings in Chronic Inflammatory Demyelinating Polyradiculoneuropathy: A Single-Center Experience
- PMID: 32560468
- PMCID: PMC7349397
- DOI: 10.3390/brainsci10060383
Pathological Findings in Chronic Inflammatory Demyelinating Polyradiculoneuropathy: A Single-Center Experience
Abstract
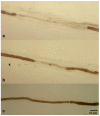
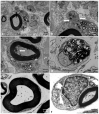
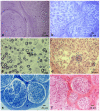
Objective: Segmental demyelination is the pathological hallmark of chronic inflammatory demyelinating polyradiculoneuropathy (CIDP), but other elementary lesions are frequently observed, configuring a series of different pathological pictures. In this article, we review the pathological findings of a large series of sural nerve biopsies from our cohort of CIDP patients.
Patients and methods: Patients with CIDP who underwent nerve biopsy were retrospectively selected from those referred to the Institute of Neurology of the "Università Cattolica del Sacro Cuore" in Rome, Italy, from 1982 to February 2020. Sural nerve biopsy was performed according to standard protocols.
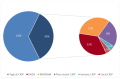
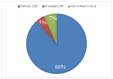
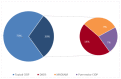
Results: Sural nerve biopsy was performed in 43/130 CIDP patients. Demyelinating abnormalities and axonal loss were found in 67.4% and 83.7% of biopsies, respectively. Conversely, onion bulbs and inflammatory infiltrates were rare (18.6% and 4.7%, respectively). In three cases, we observed normal pathological findings.
Conclusions: A pathognomonic pathological finding of CIDP cannot be established, but we confirm the utility of nerve biopsy in this setting to confirm the diagnosis (also in atypical phenotypes) and to elucidate pathogenic mechanisms.
Keywords: CIDP; axonal loss; inflammatory infiltrates; nerve biopsy; onion bulbs; regenerating clusters; segmental demyelination.
Conflict of interest statement
M.L. received financial grants (honoraria and speaking) from Ackea, Alnylam, and Pfizer, and travel grants from Ackea, Alnylam, Pfizer, Kedrion, Csl Behring, and Grifols; A.R. received travel grants from Pfizer and Csl Behring, and financial grants from Akcea; A.D.P. received travel grants from Pfizer; G.B. received financial grants (honoraria and speaking) from Alnylam, and travel grants from Pfizer, Alnylam, and Grifols; M.S. received financial grants (honoraria and speaking) from Ackea and Alnylam, and travel grants from Grifols; S.R., A.C., and F.M. have no potential conflicts of interest to disclose. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. All authors have read and agreed to the published version of the manuscript.
Figures


Similar articles
-
Diagnostic value of sural nerve demyelination in chronic inflammatory demyelinating polyneuropathy.Brain. 2001 Dec;124(Pt 12):2427-38. doi: 10.1093/brain/124.12.2427. Brain. 2001. PMID: 11701597
-
Sural nerve biopsy in chronic inflammatory demyelinating polyradiculoneuropathy.Muscle Nerve. 1989 Apr;12(4):257-64. doi: 10.1002/mus.880120402. Muscle Nerve. 1989. PMID: 2770778
-
Sural nerve biopsy in chronic inflammatory demyelinating polyneuropathy: are supportive pathologic criteria useful in diagnosis?Neurol India. 2010 Jul-Aug;58(4):542-8. doi: 10.4103/0028-3886.68673. Neurol India. 2010. PMID: 20739789
-
[Histopathological features of chronic inflammatory demyelinating polyradiculoneuropathy].Rev Neurol (Paris). 2006 Apr;162(4):527-32. doi: 10.1016/s0035-3787(06)75046-9. Rev Neurol (Paris). 2006. PMID: 16585916 Review. French.
-
History, Diagnosis, and Management of Chronic Inflammatory Demyelinating Polyradiculoneuropathy.Mayo Clin Proc. 2018 Jun;93(6):777-793. doi: 10.1016/j.mayocp.2018.03.026. Mayo Clin Proc. 2018. PMID: 29866282 Review.
Cited by
-
Relevance of Nerve Biopsy in the Diagnosis of Chronic Inflammatory Demyelinating Polyneuropathy-A Systematic Review.Diagnostics (Basel). 2022 Jul 11;12(7):1691. doi: 10.3390/diagnostics12071691. Diagnostics (Basel). 2022. PMID: 35885595 Free PMC article. Review.
-
Small Fibre Involvement in Multifocal Motor Neuropathy Explored with Sudoscan: A Single-Centre Experience.Diagnostics (Basel). 2020 Sep 26;10(10):755. doi: 10.3390/diagnostics10100755. Diagnostics (Basel). 2020. PMID: 32993111 Free PMC article.
-
New evidence for secondary axonal degeneration in demyelinating neuropathies.Neurosci Lett. 2021 Jan 23;744:135595. doi: 10.1016/j.neulet.2020.135595. Epub 2020 Dec 24. Neurosci Lett. 2021. PMID: 33359733 Free PMC article. Review.
-
Nerve Biopsy in Peripheral Neuropathies: Not All Water Is under the Bridge.Brain Sci. 2021 Apr 27;11(5):550. doi: 10.3390/brainsci11050550. Brain Sci. 2021. PMID: 33925500 Free PMC article.
-
Sex differences in Guillain Barré syndrome, chronic inflammatory demyelinating polyradiculoneuropathy and experimental autoimmune neuritis.Front Immunol. 2022 Dec 9;13:1038411. doi: 10.3389/fimmu.2022.1038411. eCollection 2022. Front Immunol. 2022. PMID: 36569912 Free PMC article. Review.
References
-
- Dyck P.J., Lais A.C., Ohta M., Bastron J.A., Okazaki H., Groover R.V. Chronic inflammatory polyradiculoneuropathy. Mayo Clin. Proc. 1975;50:621–637. - PubMed
-
- Doneddu P.E., Cocito D., Manganelli F., Fazio R., Briani C., Filosto M., Benedetti L., Mazzeo A., Marfia G.A., Cortese A., et al. Atypical CIDP: Diagnostic criteria, progression and treatment response. Data from the Italian CIDP Database. J. Neurol. Neurosurg. Psychiatry. 2019;90:125–132. doi: 10.1136/jnnp-2018-318714. - DOI - PubMed
LinkOut - more resources
Full Text Sources

