Expression Changes and Impact of the Extracellular Matrix on Etoposide Resistant Human Retinoblastoma Cell Lines
- PMID: 32560557
- PMCID: PMC7352646
- DOI: 10.3390/ijms21124322
Expression Changes and Impact of the Extracellular Matrix on Etoposide Resistant Human Retinoblastoma Cell Lines
Abstract
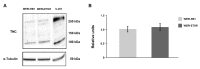
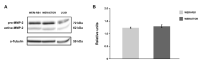
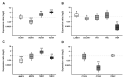
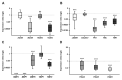
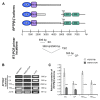
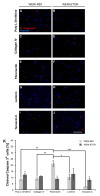
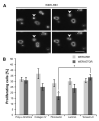
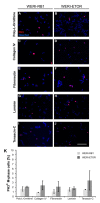
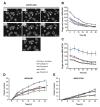
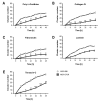
Retinoblastoma (RB) represents the most common malignant childhood eye tumor worldwide. Several studies indicate that the extracellular matrix (ECM) plays a crucial role in tumor growth and metastasis. Moreover, recent studies indicate that the ECM composition might influence the development of resistance to chemotherapy drugs. The objective of this study was to evaluate possible expression differences in the ECM compartment of the parental human cell lines WERI-RB1 (retinoblastoma 1) and Y79 and their Etoposide resistant subclones via polymerase chain reaction (PCR). Western blot analyses were performed to analyze protein levels. To explore the influence of ECM molecules on RB cell proliferation, death, and cluster formation, WERI-RB1 and resistant WERI-ETOR cells were cultivated on Fibronectin, Laminin, Tenascin-C, and Collagen IV and analyzed via time-lapse video microscopy as well as immunocytochemistry. We revealed a significantly reduced mRNA expression of the proteoglycans Brevican, Neurocan, and Versican in resistant WERI-ETOR compared to sensitive WERI-RB1 cells. Also, for the glycoproteins α1-Laminin, Fibronectin, Tenascin-C, and Tenascin-R as well as Collagen IV, reduced expression levels were observed in WERI-ETOR. Furthermore, a downregulation was detected for the matrix metalloproteinases MMP2, MMP7, MMP9, the tissue-inhibitor of metalloproteinase TIMP2, the Integrin receptor subunits ITGA4, ITGA5 and ITGB1, and all receptor protein tyrosine phosphatase β/ζ isoforms. Downregulation of Brevican, Collagen IV, Tenascin-R, MMP2, TIMP2, and ITGA5 was also verified in Etoposide resistant Y79 cells compared to sensitive ones. Protein levels of Tenascin-C and MMP-2 were comparable in both WERI cell lines. Interestingly, Fibronectin displayed an apoptosis-inducing effect on WERI-RB1 cells, whereas an anti-apoptotic influence was observed for Tenascin-C. Conversely, proliferation of WERI-ETOR cells was enhanced on Tenascin-C, while an anti-proliferative effect was observed on Fibronectin. In WERI-ETOR, cluster formation was decreased on the substrates Collagen IV, Fibronectin, and Tenascin-C. Collectively, we noted a different ECM mRNA expression and behavior of Etoposide resistant compared to sensitive RB cells. These findings may indicate a key role of ECM components in chemotherapy resistance formation of RB.
Keywords: WERI-RB1; Y79; chemotherapy resistance; etoposide; extracellular matrix; integrins; retinoblastoma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Protein Profiling of WERI-RB1 and Etoposide-Resistant WERI-ETOR Reveals New Insights into Topoisomerase Inhibitor Resistance in Retinoblastoma.Int J Mol Sci. 2022 Apr 6;23(7):4058. doi: 10.3390/ijms23074058. Int J Mol Sci. 2022. PMID: 35409416 Free PMC article.
-
Etoposide Upregulates Survival Favoring Sphingosine-1-Phosphate in Etoposide-Resistant Retinoblastoma Cells.Pathol Oncol Res. 2019 Jan;25(1):391-399. doi: 10.1007/s12253-017-0360-x. Epub 2017 Nov 15. Pathol Oncol Res. 2019. PMID: 29143233
-
Joint CB1 and NGF Receptor Activation Suppresses TRPM8 Activation in Etoposide-Resistant Retinoblastoma Cells.Int J Mol Sci. 2024 Jan 31;25(3):1733. doi: 10.3390/ijms25031733. Int J Mol Sci. 2024. PMID: 38339011 Free PMC article.
-
Tenascin-C: Form versus function.Cell Adh Migr. 2015;9(1-2):48-82. doi: 10.4161/19336918.2014.987587. Cell Adh Migr. 2015. PMID: 25482829 Free PMC article. Review.
-
[Progress in studies on effects of extracellular matrix in occurrence of proliferative vitreoretinopathy].Zhonghua Yan Ke Za Zhi. 2008 Aug;44(8):759-63. Zhonghua Yan Ke Za Zhi. 2008. PMID: 19115640 Review. Chinese.
Cited by
-
Mechanism of etoposide resistance in small cell lung cancer and the potential therapeutic options.Med Oncol. 2025 Apr 21;42(5):167. doi: 10.1007/s12032-025-02718-0. Med Oncol. 2025. PMID: 40257680
-
Extracellular matrix dynamics in tumor immunoregulation: from tumor microenvironment to immunotherapy.J Hematol Oncol. 2025 Jun 19;18(1):65. doi: 10.1186/s13045-025-01717-y. J Hematol Oncol. 2025. PMID: 40537775 Free PMC article. Review.
-
N-Glycoproteomics Study of Putative N-Glycoprotein Biomarkers of Drug Resistance in MCF-7/ADR Cells.Phenomics. 2021 Oct 28;1(6):269-284. doi: 10.1007/s43657-021-00029-8. eCollection 2021 Dec. Phenomics. 2021. PMID: 36939756 Free PMC article.
-
ADAMTS Proteases: Their Multifaceted Role in the Regulation of Cancer Metastasis.Dis Res. 2024;4(1):40-52. doi: 10.54457/DR.202401004. Dis Res. 2024. PMID: 38948119 Free PMC article.
-
Identification of dysregulation of sphingolipids in retinoblastoma using liquid chromatography-mass spectrometry.Exp Eye Res. 2024 Mar;240:109798. doi: 10.1016/j.exer.2024.109798. Epub 2024 Jan 19. Exp Eye Res. 2024. PMID: 38246332 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

