Telocytes in the Normal and Pathological Peripheral Nervous System
- PMID: 32560571
- PMCID: PMC7352954
- DOI: 10.3390/ijms21124320
Telocytes in the Normal and Pathological Peripheral Nervous System
Abstract
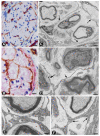
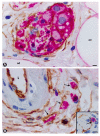
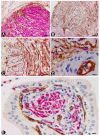
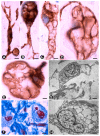
We studied telocytes/CD34+ stromal cells in the normal and pathological peripheral nervous system (PNS), for which we reviewed the literature and contributed our observations under light and electron microscopy in this field. We consider the following aspects: (A) general characteristics of telocytes and the terminology used for these cells (e.g., endoneurial stromal cells) in PNS; (B) the presence, characteristics and arrangement of telocytes in the normal PNS, including (i) nerve epi-perineurium and endoneurium (e.g., telopodes extending into the endoneurial space); (ii) sensory nerve endings (e.g., Meissner and Pacinian corpuscles, and neuromuscular spindles); (iii) ganglia; and (iv) the intestinal autonomic nervous system; (C) the telocytes in the pathologic PNS, encompassing (i) hyperplastic neurogenic processes (neurogenic hyperplasia of the appendix and gallbladder), highly demonstrative of telocyte characteristics and relations, (ii) PNS tumours, such as neurofibroma, schwannoma, granular cell tumour and nerve sheath myxoma, and interstitial cell of Cajal-related gastrointestinal stromal tumour (GIST), (iii) tumour-invaded nerves and (iv) traumatic, metabolic, degenerative or genetic neuropathies, in which there are fewer studies on telocytes, e.g., neuroinflammation and nerves in undescended testicles (cryptorchidism), Klinefelter syndrome, crush injury, mucopolysaccharidosis II (Hunter's syndrome) and Charcot-Marie-Tooth disease.
Keywords: Meissner corpuscles; appendicular neurogenic hyperplasia; gallbladder neurogenic hyperplasia; nerves; peripheral nervous system tumours; telocytes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Reappraising the microscopic anatomy of human testis: identification of telocyte networks in the peritubular and intertubular stromal space.Sci Rep. 2018 Oct 3;8(1):14780. doi: 10.1038/s41598-018-33126-2. Sci Rep. 2018. PMID: 30283023 Free PMC article.
-
Behaviour of telocytes during physiopathological activation.Semin Cell Dev Biol. 2016 Jul;55:50-61. doi: 10.1016/j.semcdb.2016.01.035. Epub 2016 Jan 27. Semin Cell Dev Biol. 2016. PMID: 26826526 Review.
-
Cd34+ Stromal Cells/Telocytes in Normal and Pathological Skin.Int J Mol Sci. 2021 Jul 8;22(14):7342. doi: 10.3390/ijms22147342. Int J Mol Sci. 2021. PMID: 34298962 Free PMC article. Review.
-
Morphological evidence of telocytes in human synovium.Sci Rep. 2018 Feb 26;8(1):3581. doi: 10.1038/s41598-018-22067-5. Sci Rep. 2018. PMID: 29483562 Free PMC article.
-
Telocytes inside of the peripheral nervous system - a 3D endoneurial network and putative role in cell communication.Rom J Morphol Embryol. 2022 Apr-Jun;63(2):335-347. doi: 10.47162/RJME.63.2.05. Rom J Morphol Embryol. 2022. PMID: 36374139 Free PMC article. Review.
Cited by
-
Gastric granular cell tumor: A case report and literature review.Oncol Lett. 2024 Jun 27;28(3):403. doi: 10.3892/ol.2024.14536. eCollection 2024 Sep. Oncol Lett. 2024. PMID: 38983126 Free PMC article.
-
A Two-Step Immunomagnetic Microbead-Based Method for the Isolation of Human Primary Skin Telocytes/CD34+ Stromal Cells.Int J Mol Sci. 2020 Aug 16;21(16):5877. doi: 10.3390/ijms21165877. Int J Mol Sci. 2020. PMID: 32824287 Free PMC article.
-
Telocytes/CD34+ Stromal Cells in Pathologically Affected White Adipose Tissue.Int J Mol Sci. 2020 Dec 18;21(24):9694. doi: 10.3390/ijms21249694. Int J Mol Sci. 2020. PMID: 33353193 Free PMC article. Review.
-
Telocytes and Other Interstitial Cells: From Structure to Function.Int J Mol Sci. 2021 May 17;22(10):5271. doi: 10.3390/ijms22105271. Int J Mol Sci. 2021. PMID: 34067777 Free PMC article.
-
Telocytes in the human ascending aorta: Characterization and exosome-related KLF-4/VEGF-A expression.J Cell Mol Med. 2021 Oct;25(20):9697-9709. doi: 10.1111/jcmm.16919. Epub 2021 Sep 25. J Cell Mol Med. 2021. PMID: 34562312 Free PMC article.
References
-
- Popescu L.M. Telocytes—A novel type of interstitial cells. In: Braisant O., Wakamatsu H., Kang I., Allegaert K., Lenbury Y., Wacholtz A., editors. Recent Researches in Modern Medicine—HISTEM. WSEAS Press; Cambridge, UK: 2011. pp. 424–432.
-
- Vannucchi M.G., Faussone-Pellegrini M.S. The telocyte subtypes. Adv. Exp. Med. Biol. 2016;913:115–126. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

