Non-optimal effectiveness of convalescent plasma transfusion and hydroxychloroquine in treating COVID-19: a case report
- PMID: 32560646
- PMCID: PMC7303939
- DOI: 10.1186/s12985-020-01354-6
Non-optimal effectiveness of convalescent plasma transfusion and hydroxychloroquine in treating COVID-19: a case report
Abstract
Background: Convalescent plasma (CP) transfusion was reported to be effective in treating critically ill patients with COVID-19, and hydroxychloroquine could potently inhibit SARS-CoV-2 in vitro. Herein, we reported a case receiving combination therapy with CP transfusion and hydroxychloroquine for the first time.
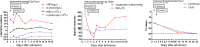
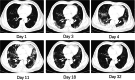
Case presentation: Laboratory findings showed high lactic acid level (2.1 mmol/L) and C-reactive protein (CRP, 48.8 mg/L), and low white blood cell count (1.96 × 109/L) in a 65-year-old Chinese man, who was diagnosed with severe COVID-19. CP was intravenously given twice, and hydroxychloroquine was orally administrated for a week (0.2 g, three times a day). The lactic acid and C-reactive protein levels remained high (2.1 mmol/L and 73.23 mg/L, respectively), while the arterial oxyhemoglobin saturation decreased to 86% with a low oxygenation index (OI, 76 mmHg) on day 4 after CP transfusion. His temperature returned to normal and the OI ascended above 300 on day 11. Moreover, the RNA test remained positive in throat swab, and computed tomography revealed severe pulmonary lesions on day 11 after admission.
Conclusion: These findings suggested that the effectiveness of combination therapy with CP and hydroxychloroquine may be non-optimal, and specific therapy needs to be explored.
Keywords: COVID-19; Convalescent plasma; Cycle threshold; Hydroxychloroquine; SARS-CoV-2.
Conflict of interest statement
All authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

