T2 and T17 cytokines alter the cargo and function of airway epithelium-derived extracellular vesicles
- PMID: 32560723
- PMCID: PMC7304225
- DOI: 10.1186/s12931-020-01402-3
T2 and T17 cytokines alter the cargo and function of airway epithelium-derived extracellular vesicles
Abstract
Background: Asthma is a common and heterogeneous disease that includes subgroups characterized by type 2 (T2) or type 17 (T17) immune responses for which there is a need to identify the underlying mechanisms and biomarkers in order to develop specific therapies. These subgroups can be defined by airway epithelium gene signatures and the airway epithelium has also been implicated to play a significant role in asthma pathology. Extracellular vesicles (EVs) carry functional biomolecules and participate in cell-to-cell communication in both health and disease, properties that are likely to be involved in airway diseases such as asthma. The aim of this study was to identify stimulus-specific proteins and functionality of bronchial epithelium-derived EVs following stimulation with T2 or T17 cytokines.
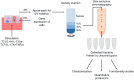
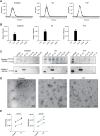
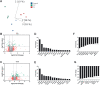
Methods: EVs from cytokine-stimulated (T2: IL-4 + IL-13 or T17: IL-17A + TNFα) human bronchial epithelial cells cultured at air-liquid interface (HBEC-ALI) were isolated by density cushion centrifugation and size exclusion chromatography and characterized with Western blotting and electron microscopy. Transcriptomic (cells) and proteomic (EVs) profiling was also performed.
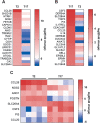
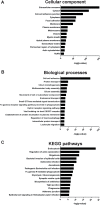
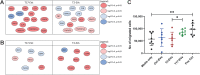
Results: Our data shows that EVs are secreted and can be isolated from the apical side of HBEC-ALI and that cytokine stimulation increases EV release. Genes upregulated in cells stimulated with T2 or T17 cytokines were increased also on protein level in the EVs. Proteins found in T17-derived EVs were suggested to be involved in pathways related to neutrophil movement which was supported by assessing neutrophil chemotaxis ex vivo.
Conclusions: Together, the results suggest that epithelial EVs are involved in airway inflammation and that the EV proteome may be used for discovery of disease-specific mechanisms and signatures which may enable a precision medicine approach to the treatment of asthma.
Keywords: Asthma; Exosomes; Mediators of inflammation; Proteomics; Respiratory epithelium.
Conflict of interest statement
E.A., Z.J. and H.O. are employees of, and hold stock/stock options, in AstraZeneca, which supported the study. C.L. and A.C. are inventors of patents using EVs as either therapeutic or diagnostics tools. C.M. and M.R. declare that they have no relevant conflicts of interest.
Figures
References
-
- Papi A, Brightling C, Pedersen SE, Reddel HK. Asthma. Lancet. 2018;391:783–800. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

