Efficacy and safety of Zihua Wenfei granules in treatment of postinfectious cough (wind-cold invading lungs syndrome): study protocol for a randomized controlled trial
- PMID: 32560739
- PMCID: PMC7304187
- DOI: 10.1186/s13063-020-04487-9
Efficacy and safety of Zihua Wenfei granules in treatment of postinfectious cough (wind-cold invading lungs syndrome): study protocol for a randomized controlled trial
Abstract
Background: Postinfectious cough usually develops and persists following respiratory tract infection. The protracted cough is embarrassing and troublesome and significantly impairs daily life. However, the optimal treatment available for this condition is still not known. This study aims to investigate the efficacy and safety of a new Chinese herbal prescription, Zihua Wenfei granule (ZHWFG), in treatment of postinfectious cough (wind-cold invading lungs syndrome).
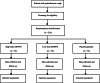
Methods: This study is a prospective, multi-center, randomized, double-blinded, parallel group, placebo-controlled trial. A total of 216 adult participants with postinfectious cough will be enrolled from six study centers across China. All participants are randomly allocated to one of three parallel treatment groups: (1) 15 g of active ZHWFG three times daily, (2) 10 g of active ZHWFG plus 5 g of ZHWFG-matched placebo three times daily, and (3) 15 g of ZHWFG-matched placebo three times daily. The treatment duration is 14 consecutive days. The primary outcomes are cough resolution rate and cough relief rate. Secondary outcomes include time to cough resolution, time to cough relief, change from baseline in cough symptom score, cough visual analog scale value, traditional Chinese medicine syndrome score at days 7 and 14, and change of CQLQ from baseline to post-treatment as well as adverse events.
Discussion: This trial may not only investigate the efficacy and safety of ZHWFG in the management of postinfectious cough (wind-cold invading lungs syndrome), but also add the evidence of Chinese herbal medicine in treatment of postinfectious cough and provide an alternative option for the management of postinfectious cough.
Trial registration: ChiCTR1900022078. Registered on 23 March 2019. http://www.chictr.org.cn/showproj.aspx?proj=36547.
Keywords: Chinese herbal medicine; Postinfectious cough; Randomized controlled trial; Study protocol; Zihua Wenfei granule.
Conflict of interest statement
The study is funded by Yuekang Pharmaceutical Co., Ltd (Beijing, China). The authors declare that there is no other conflict of interests.
Figures
Similar articles
-
Efficacy and Safety of Zihua Wenfei Zhisou Granule in Treatment of Postinfectious Cough: A Multicenter, Randomized, Double-Blind, Placebo-Controlled, Phase II Clinical Trial.Chin J Integr Med. 2025 Jan;31(1):3-10. doi: 10.1007/s11655-024-3918-y. Epub 2024 Nov 22. Chin J Integr Med. 2025. PMID: 39576445 Clinical Trial.
-
The efficacy of QingfengGanke granule in treating postinfectious cough in pathogenic wind invading lungs syndrome: a multicenter, randomized, double-blind, placebo-controlled trial.Chin Med. 2015 Aug 9;10:21. doi: 10.1186/s13020-015-0049-6. eCollection 2015. Chin Med. 2015. PMID: 26257822 Free PMC article.
-
Efficacy and safety of Tanreqing oral liquid in treatment of acute bronchitis: study protocol for a randomized controlled trial.Trials. 2022 May 7;23(1):373. doi: 10.1186/s13063-022-06318-5. Trials. 2022. PMID: 35526026 Free PMC article.
-
Chinese herbal medicine for postinfectious cough: a systematic review of randomized controlled trials.Evid Based Complement Alternat Med. 2013;2013:906765. doi: 10.1155/2013/906765. Epub 2013 Nov 20. Evid Based Complement Alternat Med. 2013. PMID: 24348727 Free PMC article. Review.
-
Effectiveness and safety of traditional Chinese herbs in children with cough variant asthma: a systematic review and Meta-analysi.J Tradit Chin Med. 2021 Oct;41(5):661-668. doi: 10.19852/j.cnki.jtcm.2021.05.001. J Tradit Chin Med. 2021. PMID: 34708623
Cited by
-
Efficacy and Safety of Zihua Wenfei Zhisou Granule in Treatment of Postinfectious Cough: A Multicenter, Randomized, Double-Blind, Placebo-Controlled, Phase II Clinical Trial.Chin J Integr Med. 2025 Jan;31(1):3-10. doi: 10.1007/s11655-024-3918-y. Epub 2024 Nov 22. Chin J Integr Med. 2025. PMID: 39576445 Clinical Trial.
References
-
- Ishida T, Yokoyama T, Iwasaku M, Saigusa M, Fukuyama H, Nakagawa H, et al. Clinical investigation of postinfectious cough among adult patients with prolonged cough. Nihon Kokyuki Gakkai Zasshi. 2010;48(3):179–185. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

