Application of yeast to studying amyloid and prion diseases
- PMID: 32560789
- PMCID: PMC7527210
- DOI: 10.1016/bs.adgen.2020.01.002
Application of yeast to studying amyloid and prion diseases
Abstract
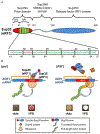
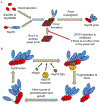
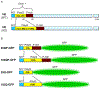
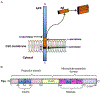
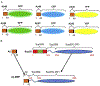
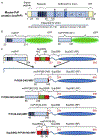
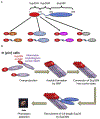
Amyloids are fibrous cross-β protein aggregates that are capable of proliferation via nucleated polymerization. Amyloid conformation likely represents an ancient protein fold and is linked to various biological or pathological manifestations. Self-perpetuating amyloid-based protein conformers provide a molecular basis for transmissible (infectious or heritable) protein isoforms, termed prions. Amyloids and prions, as well as other types of misfolded aggregated proteins are associated with a variety of devastating mammalian and human diseases, such as Alzheimer's, Parkinson's and Huntington's diseases, transmissible spongiform encephalopathies (TSEs), amyotrophic lateral sclerosis (ALS) and transthyretinopathies. In yeast and fungi, amyloid-based prions control phenotypically detectable heritable traits. Simplicity of cultivation requirements and availability of powerful genetic approaches makes yeast Saccharomyces cerevisiae an excellent model system for studying molecular and cellular mechanisms governing amyloid formation and propagation. Genetic techniques allowing for the expression of mammalian or human amyloidogenic and prionogenic proteins in yeast enable researchers to capitalize on yeast advantages for characterization of the properties of disease-related proteins. Chimeric constructs employing mammalian and human aggregation-prone proteins or domains, fused to fluorophores or to endogenous yeast proteins allow for cytological or phenotypic detection of disease-related protein aggregation in yeast cells. Yeast systems are amenable to high-throughput screening for antagonists of amyloid formation, propagation and/or toxicity. This review summarizes up to date achievements of yeast assays in application to studying mammalian and human disease-related aggregating proteins, and discusses both limitations and further perspectives of yeast-based strategies.
Keywords: Alzheimer's disease; Amyloid β; Amyotrophic lateral sclerosis; Huntington's disease; Parkinson's disease; Prion protein; Protein aggregation; Tau; Transthyretin; α-Synuclein.
© 2020 Elsevier Inc. All rights reserved.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

