Potential Roles of Redox Dysregulation in the Development of Schizophrenia
- PMID: 32560962
- PMCID: PMC7395886
- DOI: 10.1016/j.biopsych.2020.03.016
Potential Roles of Redox Dysregulation in the Development of Schizophrenia
Abstract
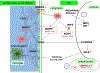
Converging evidence implicates redox dysregulation as a pathological mechanism driving the emergence of psychosis. Increased oxidative damage and decreased capacity of intracellular redox modulatory systems are consistent findings in persons with schizophrenia as well as in persons at clinical high risk who subsequently developed frank psychosis. Levels of glutathione, a key regulator of cellular redox status, are reduced in the medial prefrontal cortex, striatum, and thalamus in schizophrenia. In humans with schizophrenia and in rodent models recapitulating various features of schizophrenia, redox dysregulation is linked to reductions of parvalbumin containing gamma-aminobutyric acid (GABA) interneurons and volumes of their perineuronal nets, white matter abnormalities, and microglia activation. Importantly, the activity of transcription factors, kinases, and phosphatases regulating diverse aspects of neurodevelopment and synaptic plasticity varies according to cellular redox state. Molecules regulating interneuron function under redox control include NMDA receptor subunits GluN1 and GluN2A as well as KEAP1 (regulator of transcription factor NRF2). In a rodent schizophrenia model characterized by impaired glutathione synthesis, the Gclm knockout mouse, oxidative stress activated MMP9 (matrix metalloprotease 9) via its redox-responsive regulatory sites, causing a cascade of molecular events leading to microglia activation, perineural net degradation, and impaired NMDA receptor function. Molecular pathways under redox control are implicated in the etiopathology of schizophrenia and are attractive drug targets for individualized drug therapy trials in the contexts of prevention and treatment of psychosis.
Keywords: Clinical high risk; Gclm KO; Glutathione; Grin2A KO; MMP9; Oxidative stress; Psychosis; Redox; Schizophrenia.
Copyright © 2020 Society of Biological Psychiatry. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures
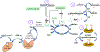
References
-
- Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J (2007): Free radicals and antioxidants in normal physiological functions and human disease. The international journal of biochemistry & cell biology. 39:44–84. - PubMed
-
- Koga M, Serritella AV, Sawa A, Sedlak TW (2016): Implications for reactive oxygen species in schizophrenia pathogenesis. Schizophr Res. 176:52–71. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

