Mineralized DNA-collagen complex-based biomaterials for bone tissue engineering
- PMID: 32561285
- PMCID: PMC7494536
- DOI: 10.1016/j.ijbiomac.2020.06.126
Mineralized DNA-collagen complex-based biomaterials for bone tissue engineering
Abstract
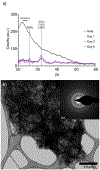
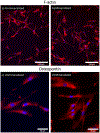
DNA is a highly polyanionic biomolecule that complexes with both collagen and hydroxyapatite. By combining these complexes, we synthesized nucleic-acid collagen complexes (NACC) mineralized with hydroxyapatite. The composite complexes were made using a short, monodisperse single-stranded DNA, type I collagen, and mineralizing medium. They rapidly self-assembled into both mineralized NACC microfibers and 3D NACC gels. At the nanoscale, these complexes are hierarchical, interwoven, curly nanofibrils resembling native extracellular matrix, which mineralized an interpenetrating nanocrystalline hydroxyapatite phase. Mineralization was able to be done either before or after NACC formation enabling temporal control of the process. In response to the NACC material, primary human osteoblasts took on an osteocyte-like morphology. Moreover, the cells agglomerated and remodeled the NACC gels into densified, tissue-like structures within 3 days. NACC fibers and gels have promise not only as osteoconductive coatings and scaffolds, but as coatings and scaffolds for any tissue using this new form of naturally-derived biomaterials.
Keywords: DNA-collagen complex; Self-assembly; Tissue engineering.
Copyright © 2020. Published by Elsevier B.V.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures








Similar articles
-
Multifunctional DNA-Collagen Biomaterials: Developmental Advances and Biomedical Applications.ACS Biomater Sci Eng. 2025 Mar 10;11(3):1253-1268. doi: 10.1021/acsbiomaterials.4c01475. Epub 2025 Jan 27. ACS Biomater Sci Eng. 2025. PMID: 39869382 Free PMC article. Review.
-
Biomimetic collagen-hydroxyapatite composite fabricated via a novel perfusion-flow mineralization technique.Tissue Eng Part C Methods. 2013 Jul;19(7):487-96. doi: 10.1089/ten.TEC.2012.0452. Epub 2013 Jan 4. Tissue Eng Part C Methods. 2013. PMID: 23157544 Free PMC article.
-
Mechanical properties and osteogenic potential of hydroxyapatite-PLGA-collagen biomaterial for bone regeneration.J Biomater Sci Polym Ed. 2016 Aug;27(11):1139-54. doi: 10.1080/09205063.2016.1184121. Epub 2016 May 12. J Biomater Sci Polym Ed. 2016. PMID: 27120980
-
Collagen-gelatin-genipin-hydroxyapatite composite scaffolds colonized by human primary osteoblasts are suitable for bone tissue engineering applications: in vitro evidences.J Biomed Mater Res A. 2014 May;102(5):1415-21. doi: 10.1002/jbm.a.34823. Epub 2013 Jun 20. J Biomed Mater Res A. 2014. PMID: 23775901
-
Mineralized collagen scaffolds for regenerative engineering applications.Curr Opin Biotechnol. 2024 Apr;86:103080. doi: 10.1016/j.copbio.2024.103080. Epub 2024 Feb 24. Curr Opin Biotechnol. 2024. PMID: 38402689 Free PMC article. Review.
Cited by
-
Gradient scaffolds in bone-soft tissue interface engineering: Structural characteristics, fabrication techniques, and emerging trends.J Orthop Translat. 2025 Jan 28;50:333-353. doi: 10.1016/j.jot.2024.10.015. eCollection 2025 Jan. J Orthop Translat. 2025. PMID: 39944791 Free PMC article. Review.
-
Programmable DNA-based biomaterials for bone tissue engineering.Fundam Res. 2025 Jan 2;5(4):1384-1400. doi: 10.1016/j.fmre.2024.12.015. eCollection 2025 Jul. Fundam Res. 2025. PMID: 40777770 Free PMC article. Review.
-
Exploring the Fibrous Nature of Single-Stranded DNA-Collagen Complexes: Nanostructural Observations and Physicochemical Insights.ACS Omega. 2024 Jul 11;9(29):32052-32058. doi: 10.1021/acsomega.4c04104. eCollection 2024 Jul 23. ACS Omega. 2024. PMID: 39072094 Free PMC article.
-
Biomineralization of Collagen-Based Materials for Hard Tissue Repair.Int J Mol Sci. 2021 Jan 19;22(2):944. doi: 10.3390/ijms22020944. Int J Mol Sci. 2021. PMID: 33477897 Free PMC article. Review.
-
Multifunctional DNA-Collagen Biomaterials: Developmental Advances and Biomedical Applications.ACS Biomater Sci Eng. 2025 Mar 10;11(3):1253-1268. doi: 10.1021/acsbiomaterials.4c01475. Epub 2025 Jan 27. ACS Biomater Sci Eng. 2025. PMID: 39869382 Free PMC article. Review.
References
-
- Izui S, Lambert PH, Miescher PA, In vitro demonstration of a particular affinity of glomerular basement membrane and collagen for DNA. A possible basis for a local formation of DNA-anti-DNA complexes in systemic lupus erythematosus., J. Exp. Med 144 (1976) 428–43. doi:10.1084/jem.144.2.428. - DOI - PMC - PubMed
-
- Rosenberg AM, Hunt DW, Petty RE, The binding of DNA to native type II collagen., J. Rheumatol 10 (1983) 925–9. http://www.ncbi.nlm.nih.gov/pubmed/6663596. - PubMed
-
- Rosenberg AM, Prokopchuk PA, The binding of dsDNA and ssDNA to human types I, II and IV collagens., J. Rheumatol 13 (1986) 512–6. http://www.ncbi.nlm.nih.gov/pubmed/3488402. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

