COVID-19 in patients with lung cancer
- PMID: 32561401
- PMCID: PMC7297689
- DOI: 10.1016/j.annonc.2020.06.007
COVID-19 in patients with lung cancer
Abstract
Background: Patients with lung cancers may have disproportionately severe coronavirus disease 2019 (COVID-19) outcomes. Understanding the patient-specific and cancer-specific features that impact the severity of COVID-19 may inform optimal cancer care during this pandemic.
Patients and methods: We examined consecutive patients with lung cancer and confirmed diagnosis of COVID-19 (n = 102) at a single center from 12 March 2020 to 6 May 2020. Thresholds of severity were defined a priori as hospitalization, intensive care unit/intubation/do not intubate ([ICU/intubation/DNI] a composite metric of severe disease), or death. Recovery was defined as >14 days from COVID-19 test and >3 days since symptom resolution. Human leukocyte antigen (HLA) alleles were inferred from MSK-IMPACT (n = 46) and compared with controls with lung cancer and no known non-COVID-19 (n = 5166).
Results: COVID-19 was severe in patients with lung cancer (62% hospitalized, 25% died). Although severe, COVID-19 accounted for a minority of overall lung cancer deaths during the pandemic (11% overall). Determinants of COVID-19 severity were largely patient-specific features, including smoking status and chronic obstructive pulmonary disease [odds ratio for severe COVID-19 2.9, 95% confidence interval 1.07-9.44 comparing the median (23.5 pack-years) to never-smoker and 3.87, 95% confidence interval 1.35-9.68, respectively]. Cancer-specific features, including prior thoracic surgery/radiation and recent systemic therapies did not impact severity. Human leukocyte antigen supertypes were generally similar in mild or severe cases of COVID-19 compared with non-COVID-19 controls. Most patients recovered from COVID-19, including 25% patients initially requiring intubation. Among hospitalized patients, hydroxychloroquine did not improve COVID-19 outcomes.
Conclusion: COVID-19 is associated with high burden of severity in patients with lung cancer. Patient-specific features, rather than cancer-specific features or treatments, are the greatest determinants of severity.
Keywords: COVID-19; chemotherapy; immunotherapy/checkpoint blockade; lung cancer; small molecule agents.
Copyright © 2020 European Society for Medical Oncology. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
Disclosure JL has received honoraria from Targeted Oncology. IRP has been a compensated consultant for Pfizer and AstraZeneca. KCA reports compensated consulting for AstraZeneca. She has received non-monetary research support from Novartis and Takeda (to her institution). JEC reports compensated consulting for AstraZeneca, Bristol-Myers Squibb, Merck, and Genentech; and research funding to institution from AstraZeneca, Bristol-Myers Squibb, Merck, and Genentech. RMD reports personal equity ownership in CVS Caremark and Roche and immediate family member with equity ownership in Pfizer, Eli Lilly, Cigna Corporation, and Baxter Bioscience. AED reports honoraria/advisory boards for Ignyta/Genentech/Roche, Loxo/Bayer/Lilly, Takeda/Ariad/Millenium, TP Therapeutics, AstraZeneca, Pfizer, Blueprint Medicines, Helsinn, Beigene, BergenBio, Hengrui Therapeutics, Exelixis, Tyra Biosciences, Verastem, MORE Health, Abbvie, 14ner/Elevation Oncology, Remedica Ltd., ArcherDX, Monopteros; associated research paid to institution from Pfizer, Exelixis, GlaxoSmithKlein, Teva, Taiho, PharmaMar; research funding from Foundation Medicine; royalties from Wolters Kluwer; other support from Merck (food/beverage), Puma (food/beverage), Merus, Boehringer Ingelheim; and CME honoraria from Medscape, OncLive, PeerVoice, Physicians Education Resources, Targeted Oncology, Research to Practice, Axis, Peerview Institute, Paradigm Medical Communications, WebMD. WVL receives institutional research funding from Daiichi Sankyo, Amgen, and Abbvie; has been a compensated consultant for PharmaMar, G1 Therapeutics, AstraZeneca, Jazz Pharmaceuticals. BTL receives institutional research funding from Genentech, Lilly, Amgen, Daiichi Sankyo, AstraZeneca, Hengrui Therapeutics, BioMedValley Discoveries, Illumina, GRAIL, Guardant Health and MORE Health; has two institutional patents at Memorial Sloan Kettering Cancer Center (US62/685,057, US62/514,661); has been a compensated consultant/advisor for Roche/Genentech, Thermo Fisher Scientific, Guardant Health, Hengrui Therapeutics, Mersana Therapeutics, and Lilly; received travel support from Resolution Bioscience and MORE Health. AN has been a compensated consultant for Bayer. MDO has been a compensated consultant for PharmMar, Novartis, and Targeted Oncology; received travel support from Bristol-Myers Squibb and Merck; received honoraria from OncLive. PKP reports honoraria/advisory boards for Boehringer Ingelheim, Celgene, EMD Serono, Celithera, AstraZeneca, Abbvie, and Lilly Oncology; was compensated for participation in an independent data safety monitoring committee for Takeda. GJR receives institutional research funding from Mirati, Merck, Pfizer, Novartis, Roche, and Takeda. CMR has been a compensated consultant regarding oncology drug development with AbbVie, Amgen, Ascentage, Astra Zeneca, Bicycle, Celgene, Daiichi-Sankyo, Genentech/Roche, Ipsen, Jazz, Lilly, Pfizer, Pharmamar, Syros, and Vavotek; serves on the scientific advisory boards of Bridge Medicines and Harpoon Therapeutics. HAY receives institutional research funding from AstraZeneca, Novartis, Pfizer, Lilly, Cullinan, and Daiichi-Sankyo; has been a compensated consultant for AstraZeneca and Daiichi-Sankyo. MGZ has received consulting fees from GlaxoSmithKline (2020), Epizyme (2017), Aldeyra Therapeutics (2019), Novocure (2019), and Atara (2018) and honoraria from Medical Learning Institute (2019) and OncLive (2019). Memorial Sloan Kettering receives research funding from the Department of Defense, the National Institutes of Health, GlaxoSmithKline, Epizyme, Polaris, Sellas Life Sciences, Bristol Myers Squibb, Millenium/Takeda, Curis, and Roche for research conducted by MGZ. MGZ serves as Chair of the Board of Directors of the Mesothelioma Applied Research Foundation. MGZ reports grants from National Institutes of Health/National Cancer Institute. Memorial Sloan Kettering has an institutional agreement with IBM for Watson for Oncology and receives royalties from IBM. MGZ is an employee of Memorial Sloan Kettering. BDG has received honoraria for speaking engagements from Merck, Bristol-Myers Squibb, and Chugai Pharmaceuticals; has been a compensated consultant for PMV Pharma and Rome Therapeutics of which he is a cofounder. MGK receives personal fees from AstraZeneca, Pfizer, Regeneron, and Daiichi-Sankyo; received honoraria for participation in educational programs from WebMD, OncLive, Physicians Education Resources, Prime Oncology, Intellisphere, Creative Educational Concepts, Peerview, i3 Health, Paradigm Medical Communications, AXIS, Carvive Systems, AstraZeneca, and Research to Practice; received travel support from AstraZeneca, Pfizer, Regeneron, and Genentech. MGK is an employee of Memorial Sloan Kettering. Memorial Sloan Kettering has received research funding from The National Cancer Institute (USA), The Lung Cancer Research Foundation, Genentech Roche, and PUMA Biotechnology for research conducted by MGK. MSK has licensed testing for EGFR T790M to MolecularMD. MDH receives institutional research funding from Bristol-Myers Squibb; has been a compensated consultant for Merck, Bristol-Myers Squibb, AstraZeneca, Genentech/Roche, Nektar, Syndax, Mirati, Shattuck Labs, Immunai, Blueprint Medicines, Achilles, and Arcus; received travel support/honoraria from AstraZeneca, Eli Lilly, and Bristol-Myers Squibb; has options from Shattuck Labs, Immunai, and Arcus; has a patent filed by his institution related to the use of tumor mutation burden to predict response to immunotherapy (PCT/US2015/062208), which has received licensing fees from PGDx. The remaining authors have declared no conflicts of interest.
Figures
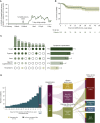


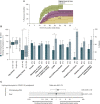
References
-
- Horn L., Whisenant J.G., Torri V., et al. Thoracic Cancers International COVID-19 Collaboration (TERAVOLT): impact of type of cancer therapy and COVID therapy on survival. J Clin Oncol. 2020;38 3818_suppl: LBA111.
-
- Luo J., Rizvi H., Egger J.V., et al. Impact of PD-1 blockade on severity of COVID-19 in patients with lung cancers. Cancer Discov. 2020 doi: 10.1158/2159-8290.CD-20-0596. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

