PACAP: A regulator of mammalian reproductive function
- PMID: 32561449
- PMCID: PMC7606562
- DOI: 10.1016/j.mce.2020.110912
PACAP: A regulator of mammalian reproductive function
Abstract
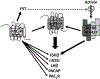
Pituitary adenylate cyclase-activating polypeptide (PACAP) is an ancestral molecule that was isolated from sheep hypothalamic extracts based on its action to stimulate cAMP production by pituitary cell cultures. PACAP is one of a number of ligands that coordinate with GnRH to control reproduction. While initially viewed as a hypothalamic releasing factor, PACAP and its receptors are widely distributed, and there is growing evidence that PACAP functions as a paracrine/autocrine regulator in the CNS, pituitary, gonads and placenta, among other tissues. This review will summarize current knowledge concerning the expression and function of PACAP in the hypothalamic-pituitary-gonadal axis with special emphasis on its role in pituitary function in the fetus and newborn.
Keywords: Hypothalamus; Ovary; Pituitary; Pituitary adenylate cyclase-activating polypeptide (PACAP); Sexual maturation; Testis.
Copyright © 2020 Elsevier B.V. All rights reserved.
Figures




References
-
- Lacombe A, Lelievre V, Roselli CE, Salameh W, Lue YH, Lawson G, Muller JM, Waschek JA, and Vilain E (2006) [A neuropeptide at the origin of testicular aging?]. Med Sci (Paris) 22, 809–811 - PubMed
-
- Shintani N, Mori W, Hashimoto H, Imai M, Tanaka K, Tomimoto S, Hirose M, Kawaguchi C, and Baba A (2002) Defects in reproductive functions in PACAP-deficient female mice. Regul Pept 109, 45–48 - PubMed
-
- Miyata A, Arimura A, Dahl RR, Minamino N, Uehara A, Jiang L, Culler MD, and Coy DH (1989) Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem Biophys Res Commun 164, 567–574 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

