Biological functions of tear film
- PMID: 32561483
- PMCID: PMC7483968
- DOI: 10.1016/j.exer.2020.108115
Biological functions of tear film
Abstract
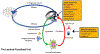
Tears have a vital function to protect and lubricate the ocular surface. Tear production, distribution and clearance is tightly regulated by the lacrimal functional unit (LFU) to meet ocular surface demands. The tear film consists of an aqueous-mucin layer, containing fluid and soluble factors produced by the lacrimal glands and mucin secreted by the goblet cells, that is covered by a lipid layer. The array of proteins, glycoproteins and lipids in tears function to maintain a stable, well-lubricated and smooth optical surface. Tear factors also promote wound healing, suppress inflammation, scavenge free radicals, and defend against microbial infection. Disease and dysfunction of the LFU leads to tear instability, increased evaporation, inflammation, and blurred and fluctuating vision. The function of tear components and the consequences of tear deficiency on the ocular surface are reviewed.
Keywords: Dry eye; Dry eye disease; Growth factor; Innate immunity; Lipid; Mucin; Pain; Tear stability; Tears; Visual acuity.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Disclosure statement: None of the authors have any financial or personal relationships to disclose that would cause a conflict of interest regarding this article.
Figures
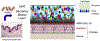
References
-
- Argueso P, Balaram M, Spurr-Michaud S, Keutmann HT, Dana MR, Gipson IK, 2002. Decreased levels of the goblet cell mucin MUC5AC in tears of patients with Sjogren syndrome. Invest Ophthalmol Vis Sci 43, 1004–1011. - PubMed
-
- Bagga B, Motukupally SR, Mohamed A, 2018. Microbial keratitis in Stevens-Johnson syndrome: Clinical and microbiological profile. Ocul Surf 16, 454–457. - PubMed
-
- Behrens A, Doyle JJ, Stern L, Chuck RS, McDonnell PJ, Azar DT, Dua HS, Hom M, Karpecki PM, Laibson PR, Lemp MA, Meisler DM, Del Castillo JM, O’Brien TP, Pflugfelder SC, Rolando M, Schein OD, Seitz B, Tseng SC, van Setten G, Wilson SE, Yiu SC, 2006. Dysfunctional tear syndrome: a Delphi approach to treatment recommendations. Cornea 25, 900–907. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

