Characterization of a Knock-In Mouse Line Expressing a Fusion Protein of κ Opioid Receptor Conjugated with tdTomato: 3-Dimensional Brain Imaging via CLARITY
- PMID: 32561573
- PMCID: PMC7385665
- DOI: 10.1523/ENEURO.0028-20.2020
Characterization of a Knock-In Mouse Line Expressing a Fusion Protein of κ Opioid Receptor Conjugated with tdTomato: 3-Dimensional Brain Imaging via CLARITY
Abstract
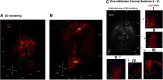
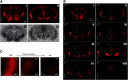
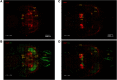
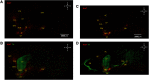
Activation of κ opioid receptor (KOR) produces analgesia, antipruritic effect, sedation and dysphoria. To characterize neuroanatomy of KOR at high resolutions and circumvent issues of specificity of KOR antibodies, we generated a knock-in mouse line expressing KOR fused at the C terminus with the fluorescent protein tdTomato (KtdT). The selective KOR agonist U50,488H caused anti-scratch effect and hypolocomotion, indicating intact KOR neuronal circuitries. Clearing of brains with CLARITY revealed three-dimensional (3-D) images of distribution of KOR, and any G-protein-coupled receptors, for the first time. 3-D brain images of KtdT and immunohistochemistry (IHC) on brain sections with antibodies against tdTomato show similar distribution to that of autoradiography of [3H]U69,593 binding to KOR in wild-type mice. KtdT was observed in regions involved in reward and aversion, pain modulation, and neuroendocrine regulation. KOR is present in several areas with unknown roles, including the claustrum (CLA), dorsal endopiriform nucleus, paraventricular nucleus of the thalamus (PVT), lateral habenula (LHb), and substantia nigra pars reticulata (SNr), which are discussed. Prominent KtdT-containing fibers were observed to project from caudate putamen (CP) and nucleus accumbens (ACB) to substantia innominata (SI) and SNr. Double IHC revealed co-localization of KtdT with tyrosine hydroxylase (TH) in brain regions, including CP, ACB, and ventral tegmental area (VTA). KOR was visualized at the cellular level, such as co-localization with TH and agonist-induced KOR translocation into intracellular space in some VTA neurons. These mice thus represent a powerful and heretofore unparalleled tool for neuroanatomy of KOR at both the 3-D and cellular levels.
Keywords: 3-D imaging; CLARITY; neuroanatomy; κ opioid receptor.
Copyright © 2020 Chen et al.
Figures


References
-
- Arvidsson U, Riedl M, Chakrabarti S, Vulchanova L, Lee JH, Nakano AH, Lin X, Loh HH, Law PY, Wessendorf MW (1995) The kappa-opioid receptor is primarily postsynaptic: combined immunohistochemical localization of the receptor and endogenous opioids. Proc Natl Acad Sci USA 92:5062–5066. 10.1073/pnas.92.11.5062 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
