Insights into the Maturation of Pernisine, a Subtilisin-Like Protease from the Hyperthermophilic Archaeon Aeropyrum pernix
- PMID: 32561587
- PMCID: PMC7440795
- DOI: 10.1128/AEM.00971-20
Insights into the Maturation of Pernisine, a Subtilisin-Like Protease from the Hyperthermophilic Archaeon Aeropyrum pernix
Abstract
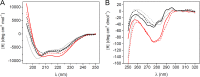
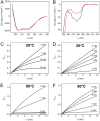
Pernisine is a subtilisin-like protease that was originally identified in the hyperthermophilic archaeon Aeropyrum pernix, which lives in extreme marine environments. Pernisine shows exceptional stability and activity due to the high-temperature conditions experienced by A. pernix Pernisine is of interest for industrial purposes, as it is one of the few proteases that has demonstrated prion-degrading activity. Like other extracellular subtilisins, pernisine is synthesized in its inactive pro-form (pro-pernisine), which needs to undergo maturation to become proteolytically active. The maturation processes of mesophilic subtilisins have been investigated in detail; however, less is known about the maturation of their thermophilic homologs, such as pernisine. Here, we show that the structure of pro-pernisine is disordered in the absence of Ca2+ ions. In contrast to the mesophilic subtilisins, pro-pernisine requires Ca2+ ions to adopt the conformation suitable for its subsequent maturation. In addition to several Ca2+-binding sites that have been conserved from the thermostable Tk-subtilisin, pernisine has an additional insertion sequence with a Ca2+-binding motif. We demonstrate the importance of this insertion for efficient folding and stabilization of pernisine during its maturation. Moreover, analysis of the pernisine propeptide explains the high-temperature requirement for pro-pernisine maturation. Of note, the propeptide inhibits the pernisine catalytic domain more potently at high temperatures. After dissociation, the propeptide is destabilized at high temperatures only, which leads to its degradation and finally to pernisine activation. Our data provide new insights into and understanding of the thermostable subtilisin autoactivation mechanism.IMPORTANCE Enzymes from thermophilic organisms are of particular importance for use in industrial applications, due to their exceptional stability and activity. Pernisine, from the hyperthermophilic archaeon Aeropyrum pernix, is a proteolytic enzyme that can degrade infective prion proteins and thus has a potential use for disinfection of prion-contaminated surfaces. Like other subtilisin-like proteases, pernisine needs to mature through an autocatalytic process to become an active protease. In the present study, we address the maturation of pernisine and show that the process is regulated specifically at high temperatures by the propeptide. Furthermore, we demonstrate the importance of a unique Ca2+-binding insertion for stabilization of mature pernisine. Our results provide a novel understanding of thermostable subtilisin autoactivation, which might advance the development of these enzymes for commercial use.
Keywords: Aeropyrum pernix; Ca2+ binding; maturation; pernisine; propeptide; subtilisin.
Copyright © 2020 American Society for Microbiology.
Figures








References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

