Label-free hematology analysis using deep-ultraviolet microscopy
- PMID: 32561645
- PMCID: PMC7334528
- DOI: 10.1073/pnas.2001404117
Label-free hematology analysis using deep-ultraviolet microscopy
Abstract
Hematological analysis, via a complete blood count (CBC) and microscopy, is critical for screening, diagnosing, and monitoring blood conditions and diseases but requires complex equipment, multiple chemical reagents, laborious system calibration and procedures, and highly trained personnel for operation. Here we introduce a hematological assay based on label-free molecular imaging with deep-ultraviolet microscopy that can provide fast quantitative information of key hematological parameters to facilitate and improve hematological analysis. We demonstrate that this label-free approach yields 1) a quantitative five-part white blood cell differential, 2) quantitative red blood cell and hemoglobin characterization, 3) clear identification of platelets, and 4) detailed subcellular morphology. Analysis of tens of thousands of live cells is achieved in minutes without any sample preparation. Finally, we introduce a pseudocolorization scheme that accurately recapitulates the appearance of cells under conventional staining protocols for microscopic analysis of blood smears and bone marrow aspirates. Diagnostic efficacy is evaluated by a panel of hematologists performing a blind analysis of blood smears from healthy donors and thrombocytopenic and sickle cell disease patients. This work has significant implications toward simplifying and improving CBC and blood smear analysis, which is currently performed manually via bright-field microscopy, and toward the development of a low-cost, easy-to-use, and fast hematological analyzer as a point-of-care device and for low-resource settings.
Keywords: deep-UV microscopy; hematology analysis; label-free cell classification; molecular imaging; point-of-care diagnosis.
Conflict of interest statement
The authors declare no competing interest.
Figures



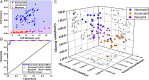


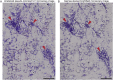
References
-
- Honda T., Uehara T., Matsumoto G., Arai S., Sugano M., Neutrophil left shift and white blood cell count as markers of bacterial infection. Clin. Chim. Acta 457, 46–53 (2016). - PubMed
-
- Velo-García A., Castro S. G., Isenberg D. A., The diagnosis and management of the haematologic manifestations of lupus. J. Autoimmun. 74, 139–160 (2016). - PubMed
-
- Crawford J., Dale D. C., Lyman G. H., Chemotherapy-induced neutropenia: Risks, consequences, and new directions for its management. Cancer 100, 228–237 (2004). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

