A role for NPY-NPY2R signaling in albuminuric kidney disease
- PMID: 32561647
- PMCID: PMC7355002
- DOI: 10.1073/pnas.2004651117
A role for NPY-NPY2R signaling in albuminuric kidney disease
Abstract
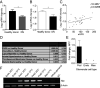
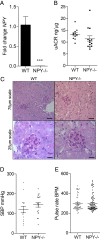
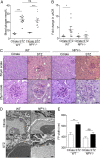
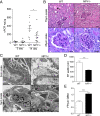
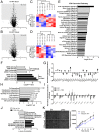
Albuminuria is an independent risk factor for the progression to end-stage kidney failure, cardiovascular morbidity, and premature death. As such, discovering signaling pathways that modulate albuminuria is desirable. Here, we studied the transcriptomes of podocytes, key cells in the prevention of albuminuria, under diabetic conditions. We found that Neuropeptide Y (NPY) was significantly down-regulated in insulin-resistant vs. insulin-sensitive mouse podocytes and in human glomeruli of patients with early and late-stage diabetic nephropathy, as well as other nondiabetic glomerular diseases. This contrasts with the increased plasma and urinary levels of NPY that are observed in such conditions. Studying NPY-knockout mice, we found that NPY deficiency in vivo surprisingly reduced the level of albuminuria and podocyte injury in models of both diabetic and nondiabetic kidney disease. In vitro, podocyte NPY signaling occurred via the NPY2 receptor (NPY2R), stimulating PI3K, MAPK, and NFAT activation. Additional unbiased proteomic analysis revealed that glomerular NPY-NPY2R signaling predicted nephrotoxicity, modulated RNA processing, and inhibited cell migration. Furthermore, pharmacologically inhibiting the NPY2R in vivo significantly reduced albuminuria in adriamycin-treated glomerulosclerotic mice. Our findings suggest a pathogenic role of excessive NPY-NPY2R signaling in the glomerulus and that inhibiting NPY-NPY2R signaling in albuminuric kidney disease has therapeutic potential.
Copyright © 2020 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures

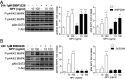
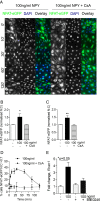
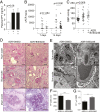
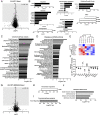
References
-
- Eckardt K. U., et al., Evolving importance of kidney disease: From subspecialty to global health burden. Lancet 382, 158–169 (2013). - PubMed
-
- Mogensen C. E., Microalbuminuria predicts clinical proteinuria and early mortality in maturity-onset diabetes. N. Engl. J. Med. 310, 356–360 (1984). - PubMed
-
- Tryggvason K., Patrakka J., Wartiovaara J., Hereditary proteinuria syndromes and mechanisms of proteinuria. N. Engl. J. Med. 354, 1387–1401 (2006). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

