Germline mutation rates in young adults predict longevity and reproductive lifespan
- PMID: 32561805
- PMCID: PMC7305191
- DOI: 10.1038/s41598-020-66867-0
Germline mutation rates in young adults predict longevity and reproductive lifespan
Abstract
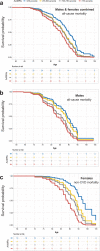
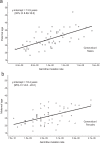
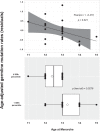
Ageing may be due to mutation accumulation across the lifespan, leading to tissue dysfunction, disease, and death. We tested whether germline autosomal mutation rates in young adults predict their remaining survival, and, for women, their reproductive lifespans. Age-adjusted mutation rates (AAMRs) in 61 women and 61 men from the Utah CEPH (Centre d'Etude du Polymorphisme Humain) families were determined. Age at death, cause of death, all-site cancer incidence, and reproductive histories were provided by the Utah Population Database, Utah Cancer Registry, and Utah Genetic Reference Project. Higher AAMRs were significantly associated with higher all-cause mortality in both sexes combined. Subjects in the top quartile of AAMRs experienced more than twice the mortality of bottom quartile subjects (hazard ratio [HR], 2.07; 95% confidence interval [CI], 1.21-3.56; p = 0.008; median survival difference = 4.7 years). Fertility analyses were restricted to women whose age at last birth (ALB) was ≥ 30 years, the age when fertility begins to decline. Women with higher AAMRs had significantly fewer live births and a younger ALB. Adult germline mutation accumulation rates are established in adolescence, and later menarche in women is associated with delayed mutation accumulation. We conclude that germline mutation rates in healthy young adults may provide a measure of both reproductive and systemic ageing. Puberty may induce the establishment of adult mutation accumulation rates, just when DNA repair systems begin their lifelong decline.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Holmes GE, Bernstein C, Bernstein H. Oxidative and other DNA damages as the basis of aging: a review. Mutat. Res. 1992;275:305–315. - PubMed
-
- Navarro CL, Cau P, Lévy N. Molecular bases of progeroid syndromes. Hum. Mol. Genet. 2006;15:R151–161. - PubMed
-
- Martin GM, et al. Somatic mutations are frequent and increase with age in human kidney epithelial cells. Hum. Mol. Genet. 1996;5:215–221. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

