Antibiotic effect and microbiome persistence vary along the European seabass gut
- PMID: 32561815
- PMCID: PMC7305304
- DOI: 10.1038/s41598-020-66622-5
Antibiotic effect and microbiome persistence vary along the European seabass gut
Abstract
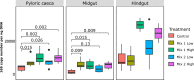
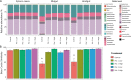
The constant increase in aquaculture production has led to extensive use of antibiotics as a means to prevent and treat diseases, with adverse implications on the environment, animal health and commensal microbes. Gut microbes are important for the host proper functioning, thus evaluating such impacts is highly crucial. Examining the antibiotic impact on gut segments with different physiological roles may provide insight into their effects on these microhabitats. Hence, we evaluated the effect of feed-administrated antibiotics on the composition and metabolic potential of the gut microbiome in the European seabass, an economically important aquaculture species. We used quantitative PCR to measure bacterial copy numbers, and amplicon sequencing of the 16S rRNA gene to describe the composition along the gut, after 7-days administration of two broad-range antibiotic mixtures at two concentrations. While positive correlation was found between antibiotic concentration and bacterial abundance, we showed a differential effect of antibiotics on the composition along the gut, highlighting distinct impacts on these microbial niches. Moreover, we found an increase in abundance of predicted pathways related to antibiotic-resistance. Overall, we show that a high portion of the European seabass gut microbiome persisted, despite the examined antibiotic intake, indicating high stability to perturbations.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- FAO. The State of World Fisheries and Aquaculture 2018 - Meeting the sustainable development goals. Rome. Licence: CC BY-NC-SA 3.0 IGO. (2018).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

