In situ detection of SARS-CoV-2 in lungs and airways of patients with COVID-19
- PMID: 32561849
- PMCID: PMC7304376
- DOI: 10.1038/s41379-020-0595-z
In situ detection of SARS-CoV-2 in lungs and airways of patients with COVID-19
Abstract
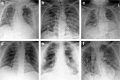
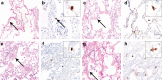
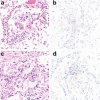
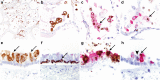
Coronavirus disease 2019 (COVID-19) caused by SARS-CoV-2 has led to a global public health crisis. In elderly individuals and those with comorbidities, COVID-19 is associated with high mortality, frequently caused by acute respiratory distress syndrome. We examine in situ expression of SARS-CoV-2 in airways and lung obtained at autopsy of individuals with confirmed COVID-19 infection. Seven autopsy cases (male, N = 5; female, N = 2) with reverse transcriptase-polymerase chain reaction (RT-PCR)-confirmed SARS-CoV-2 infection and a median age of 66 years (range, 50-77 years) were evaluated using a rabbit polyclonal antibody against SARS Nucleocapsid protein in correlation with clinical parameters. The median time from symptom onset to death was 9 days (range, 6-31 days), from hospitalization 7 days (range, 1-21 days), from positive RT-PCR 7 days (range, 0-18 days), and from intensive care unit admission defining onset of respiratory failure 3 days (range, 1-18 days). Chest imaging identified diffuse airspace disease in all patients corresponding to acute and (N = 5) or organizing (N = 2) diffuse alveolar damage (DAD) on histologic examination. Among five patients with acute-phase DAD (≤7 days from onset of respiratory failure), SARS-CoV-2 was detected in pulmonary pneumocytes and ciliated airway cells (N = 5), and in upper airway epithelium (N = 2). In two patients with organizing DAD (>14 days from onset of respiratory failure), no virus was detected in lungs or airways. No endothelial cell infection was observed. The findings suggest that SARS-CoV-2 infection of epithelial cells in lungs and airways of patients with COVID-19 who developed respiratory failure can be detected during the acute phase of lung injury and is absent in the organizing phase.
Figures



References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

