Wnt/β-catenin activation cooperates with loss of p53 to cause adrenocortical carcinoma in mice
- PMID: 32561853
- PMCID: PMC7378041
- DOI: 10.1038/s41388-020-1358-5
Wnt/β-catenin activation cooperates with loss of p53 to cause adrenocortical carcinoma in mice
Abstract
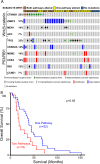
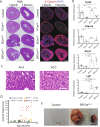
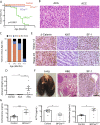
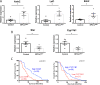
Adrenocortical carcinoma (ACC) is a rare and aggressive malignancy with limited therapeutic options. The lack of mouse models that recapitulate the genetics of ACC has hampered progress in the field. We analyzed The Cancer Genome Atlas (TCGA) dataset for ACC and found that patients harboring alterations in both p53/Rb and Wnt/β-catenin signaling pathways show a worse prognosis compared with patients that harbored alterations in only one. To model this, we utilized the Cyp11b2(AS)Cre mouse line to generate mice with adrenocortical-specific Wnt/β-catenin activation, Trp53 deletion, or the combination of both. Mice with targeted Wnt/β-catenin activation or Trp53 deletion showed no changes associated with tumor formation. In contrast, alterations in both pathways led to ACC with pulmonary metastases. Similar to ACCs in humans, these tumors produced increased levels of corticosterone and aldosterone and showed a high proliferation index. Gene expression analysis revealed that mouse tumors exhibited downregulation of Star and Cyp11b1 and upregulation of Ezh2, similar to ACC patients with a poor prognosis. Altogether, these data show that altering both Wnt/β-catenin and p53/Rb signaling is sufficient to drive ACC in mouse. This autochthonous model of ACC represents a new tool to investigate the biology of ACC and to identify new treatment strategies.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
-
- Fassnacht M, Kroiss M, Allolio B. Update in adrenocortical carcinoma. J Clin Endocrinol Metab. 2013;98:4551–64. - PubMed
-
- Fassnacht M, Dekkers O, Else T, Baudin E, Berruti A, de Krijger RR, et al. European Society of Endocrinology Clinical Practice Guidelines on the Management of Adrenocortical Carcinoma in Adults, in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol. 2018;179:G1–46. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

