The adjuvant effect of melanin is superior to incomplete Freund's adjuvant in subunit/peptide vaccines in mice
- PMID: 32561966
- PMCID: PMC11027463
- DOI: 10.1007/s00262-020-02631-7
The adjuvant effect of melanin is superior to incomplete Freund's adjuvant in subunit/peptide vaccines in mice
Abstract
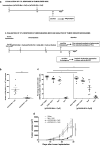
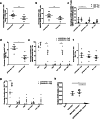
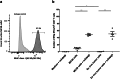
Peptide vaccines represent an attractive alternative to conventional anti-tumor therapies, but have not yet achieved significant clinical efficacy with commonly used formulations. Combination of short antigenic peptides, synthetic melanin and TLR9 agonist (Toll-like receptor 9, CpG-28) was reported as highly efficient to trigger strong CD8 + T-cell responses. We compared this vaccine approach to the standard adjuvant formulation that combines the incomplete Freund's adjuvant (IFA) and CpG-28, using either an ovalbumin epitope (pOVA30) or a spontaneously occurring tumor neoepitope (mAdpgk).Melanin-based vaccine induced significantly higher cytotoxic T lymphocytes (CTL) responses than IFA-based vaccine in both pOVA30- and mAdpgk-targeted vaccines. The anti-tumor efficacy of melanin-based vaccine was further assessed in mice, grafted either with E.G7-OVA cells (E.G7 cells transfected with ovalbumin) or with MC38 cells that spontaneously express the mAdpgk neoepitope. Melanin-based vaccine induced a major inhibition of E.G7-OVA tumor growth when compared to IFA-based vaccine (p < 0.001), but tumors eventually relapsed from day 24. In the MC38 tumor model, no significant inhibition of tumor growth was observed. In both cases, tumor escape appeared related to the loss of antigen presentation by tumor cells (loss of ovalbumin expression in E.G7-OVA model; poor presentation of mAdpgk in MC38 model), although the CTL responses displayed an effector memory phenotype, a high cytolytic potential and low programmed cell death-1 (PD1) expression.In conclusion, synthetic melanin can be efficiently used as an adjuvant to enhance T-cells response against subunit vaccine antigens and compared favorably to the classic combination of IFA and TLR9 agonist in mice.
Keywords: Cancer vaccine; Immunotherapy; Melanin; Neoepitope; PD1.
Conflict of interest statement
The AP/HP (Assistance Publique de Hopitaux de Paris) filed a provisional patent application on this method. AF Carpentier & C Banissi are listed as inventors. AF Carpentier holds shares in Altevax inc. and is consultant for BMS. The authors declare that there are no other conflicts of interest.
Figures

Similar articles
-
Incomplete Freund's adjuvant reduces arginase and enhances Th1 dominance, TLR signaling and CD40 ligand expression in the vaccine site microenvironment.J Immunother Cancer. 2020 Apr;8(1):e000544. doi: 10.1136/jitc-2020-000544. J Immunother Cancer. 2020. PMID: 32350119 Free PMC article.
-
Diaminosulfide based polymer microparticles as cancer vaccine delivery systems.J Control Release. 2015 Dec 28;220(Pt B):682-90. doi: 10.1016/j.jconrel.2015.09.002. Epub 2015 Sep 8. J Control Release. 2015. PMID: 26359124 Free PMC article.
-
Simultaneous CD8+ T cell responses to multiple tumor antigen epitopes in a multipeptide melanoma vaccine.Cancer Immun. 2003 Oct 28;3:15. Cancer Immun. 2003. PMID: 14580186
-
Peptide emulsions in incomplete Freund's adjuvant create effective nurseries promoting egress of systemic CD4+ and CD8+ T cells for immunotherapy of cancer.J Immunother Cancer. 2022 Sep;10(9):e004709. doi: 10.1136/jitc-2022-004709. J Immunother Cancer. 2022. PMID: 36939214 Free PMC article. Review.
-
The Changing Landscape of Therapeutic Cancer Vaccines-Novel Platforms and Neoantigen Identification.Clin Cancer Res. 2021 Feb 1;27(3):689-703. doi: 10.1158/1078-0432.CCR-20-0245. Epub 2020 Oct 29. Clin Cancer Res. 2021. PMID: 33122346 Review.
Cited by
-
Applications of Melanin and Melanin-Like Nanoparticles in Cancer Therapy: A Review of Recent Advances.Cancers (Basel). 2021 Mar 23;13(6):1463. doi: 10.3390/cancers13061463. Cancers (Basel). 2021. PMID: 33806772 Free PMC article. Review.
-
Immune Response Activation and Hepatoprotective Activity of Randia echinocarpa Soluble Melanins in Murine Models.Int J Food Sci. 2025 Apr 14;2025:5888390. doi: 10.1155/ijfo/5888390. eCollection 2025. Int J Food Sci. 2025. PMID: 40259921 Free PMC article.
-
Melanin-like nanoparticles: advances in surface modification and tumour photothermal therapy.J Nanobiotechnology. 2022 Nov 19;20(1):485. doi: 10.1186/s12951-022-01698-x. J Nanobiotechnology. 2022. PMID: 36402976 Free PMC article. Review.
-
Synthetic Melanin Acts as Efficient Peptide Carrier in Cancer Vaccine Strategy.Int J Mol Sci. 2022 Nov 29;23(23):14975. doi: 10.3390/ijms232314975. Int J Mol Sci. 2022. PMID: 36499300 Free PMC article.
-
Cancer Vaccines: Adjuvant Potency, Importance of Age, Lifestyle, and Treatments.Front Immunol. 2021 Feb 17;11:615240. doi: 10.3389/fimmu.2020.615240. eCollection 2020. Front Immunol. 2021. PMID: 33679703 Free PMC article. Review.
References
-
- Carpentier AF, Tran T, Sejalon F, Geinguenaud F, Tartour E, Motte L, Banissi C. The adjuvant effect of melanin is superior to incomplete Freund adjuvant in a tumor subunit vaccine model, Eur J Cancer, March 2018 Volume 92, Supplement 1, p S2–S3 [Abstract A4]
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

