CB1 receptor-dependent desensitisation of TRPV1 channels contributes to the analgesic effect of dipyrone in sensitised primary sensory neurons
- PMID: 32562269
- PMCID: PMC7520441
- DOI: 10.1111/bph.15170
CB1 receptor-dependent desensitisation of TRPV1 channels contributes to the analgesic effect of dipyrone in sensitised primary sensory neurons
Abstract
Background and purpose: While dipyrone is a widely used analgesic, its mechanism of action is not completely understood. Recently, we have reported that the dipyrone metabolite 4-aminoantipyrine (4-AA) reduces PGE2 -induced pain-related behaviour through cannabinoid CB1 receptors. Here, we ascertained, in naive and PGE2 -induced "inflamed" conditions, both in vivo and in vitro, the molecular mechanisms involved in the 4-AA-induced analgesic effects.
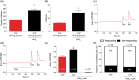
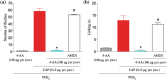
Experimental approach: The effect of local administration of 4-AA (160 μg per paw) on capsaicin (0.12 μg per paw) injection-induced pain-related behaviour and 4-AA's effect on 500-nM capsaicin-induced changes in intracellular calcium concentration ([Ca2+ ]i ) in cultured primary sensory neurons were assessed in vivo and in vitro, respectively.
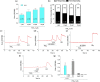
Key results: 4-AA reduced capsaicin-induced nociceptive behaviour in naive and inflamed conditions through CB1 receptors. 4-AA (100 μM) reduced capsaicin-induced increase in [Ca2+ ]i in a CB1 receptor-dependent manner, when PGE2 was not present. Following PGE2 application, 4-AA (1-50 μM) increased the [Ca2+ ]i . Although 4-AA activated both TRPV1 and TRPA1 channels, increased [Ca2+ ]i was mediated through TRPV1 channels. Activation of TRPV1 channels resulted in their desensitisation. Blocking CB1 receptors reduced both the excitatory and desensitising effects of 4-AA.
Conclusion and implications: CB1 receptor-mediated inhibition of TRPV1 channels and TRPV1-mediated Ca2+ -influx- and CB1 receptor-dependent desensitisation of TRPV1 channels contribute to the anti-nociceptive effect of 4-AA in naive and inflamed conditions respectively. Agonists active at both CB1 receptors and TRPV1 channels might be useful as analgesics, particularly in inflammatory conditions.
Keywords: CB1; TRPA1; TRPV1; calcium imaging; dipyrone; dorsal root ganglion.
© 2020 The British Pharmacological Society.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Ahluwalia, J. , Urban, L. , Bevan, S. , & Nagy, I. (2003). Anandamide regulates neuropeptide release from capsaicin‐sensitive primary sensory neurons by activating both the cannabinoid 1 receptor and the vanilloid receptor 1 in vitro. The European Journal of Neuroscience, 17, 2611–2618. 10.1046/j.1460-9568.2003.02703.x - DOI - PubMed
-
- Ahluwalia, J. , Urban, L. , Capogna, M. , Bevan, S. , and Nagy, I. (2000). Cannabinoid 1 receptors are expressed in nociceptive primary sensory neurons. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

