A proposed role for the SARS-CoV-2 nucleocapsid protein in the formation and regulation of biomolecular condensates
- PMID: 32562316
- PMCID: PMC7323129
- DOI: 10.1096/fj.202001351
A proposed role for the SARS-CoV-2 nucleocapsid protein in the formation and regulation of biomolecular condensates
Abstract
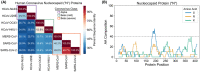
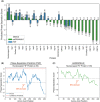
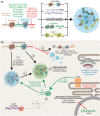
To date, the recently discovered SARS-CoV-2 virus has afflicted >6.9 million people worldwide and disrupted the global economy. Development of effective vaccines or treatments for SARS-CoV-2 infection will be aided by a molecular-level understanding of SARS-CoV-2 proteins and their interactions with host cell proteins. The SARS-CoV-2 nucleocapsid (N) protein is highly homologous to the N protein of SARS-CoV, which is essential for viral RNA replication and packaging into new virions. Emerging models indicate that nucleocapsid proteins of other viruses can form biomolecular condensates to spatiotemporally regulate N protein localization and function. Our bioinformatic analyses, in combination with pre-existing experimental evidence, suggest that the SARS-CoV-2 N protein is capable of forming or regulating biomolecular condensates in vivo by interaction with RNA and key host cell proteins. We discuss multiple models, whereby the N protein of SARS-CoV-2 may harness this activity to regulate viral life cycle and host cell response to viral infection.
Keywords: enveloped viruses; liquid-liquid phase separation; low-complexity domain; stress granule; viral capsid.
© 2020 The Authors. The FASEB Journal published by Wiley Periodicals LLC on behalf of Federation of American Societies for Experimental Biology.
Conflict of interest statement
The authors declare that they have no conflicting interests.
Figures
Similar articles
-
SARS-CoV-2 nucleocapsid protein phase-separates with RNA and with human hnRNPs.EMBO J. 2020 Dec 15;39(24):e106478. doi: 10.15252/embj.2020106478. Epub 2020 Dec 4. EMBO J. 2020. PMID: 33200826 Free PMC article.
-
Phase Separation of SARS-CoV-2 Nucleocapsid Protein with TDP-43 Is Dependent on C-Terminus Domains.Int J Mol Sci. 2024 Aug 12;25(16):8779. doi: 10.3390/ijms25168779. Int J Mol Sci. 2024. PMID: 39201466 Free PMC article.
-
The SARS-CoV-2 nucleocapsid phosphoprotein forms mutually exclusive condensates with RNA and the membrane-associated M protein.Nat Commun. 2021 Jan 21;12(1):502. doi: 10.1038/s41467-020-20768-y. Nat Commun. 2021. PMID: 33479198 Free PMC article.
-
Liquid-liquid phase separation of nucleocapsid proteins during SARS-CoV-2 and HIV-1 replication.Cell Rep. 2023 Jan 31;42(1):111968. doi: 10.1016/j.celrep.2022.111968. Epub 2022 Dec 26. Cell Rep. 2023. PMID: 36640305 Free PMC article. Review.
-
Structural basis for the participation of the SARS-CoV-2 nucleocapsid protein in the template switch mechanism and genomic RNA reorganization.J Biol Chem. 2024 Nov;300(11):107834. doi: 10.1016/j.jbc.2024.107834. Epub 2024 Sep 27. J Biol Chem. 2024. PMID: 39343000 Free PMC article. Review.
Cited by
-
Publishing coronavirology: Peering into peer(less?) review.FASEB J. 2020 Aug;34(8):9825-9827. doi: 10.1096/fj.202001592. FASEB J. 2020. PMID: 32803811 Free PMC article. No abstract available.
-
Signaling mechanisms of SARS-CoV-2 Nucleocapsid protein in viral infection, cell death and inflammation.Int J Biol Sci. 2022 Jul 11;18(12):4704-4713. doi: 10.7150/ijbs.72663. eCollection 2022. Int J Biol Sci. 2022. PMID: 35874957 Free PMC article. Review.
-
SARS-CoV-2-Specific T Cell Immunity in HIV-Associated Kaposi Sarcoma Patients in Zambia.J Immunol Res. 2022 Jul 28;2022:2114285. doi: 10.1155/2022/2114285. eCollection 2022. J Immunol Res. 2022. PMID: 35935575 Free PMC article.
-
How does severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) achieve immune evasion?: A narrative review.Medicine (Baltimore). 2024 Apr 19;103(16):e37780. doi: 10.1097/MD.0000000000037780. Medicine (Baltimore). 2024. PMID: 38640329 Free PMC article. Review.
-
Inhibition of anti-viral stress granule formation by coronavirus endoribonuclease nsp15 ensures efficient virus replication.PLoS Pathog. 2021 Feb 26;17(2):e1008690. doi: 10.1371/journal.ppat.1008690. eCollection 2021 Feb. PLoS Pathog. 2021. PMID: 33635931 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

