Diffusion Imaging in the Post HCP Era
- PMID: 32562456
- PMCID: PMC8647969
- DOI: 10.1002/jmri.27247
Diffusion Imaging in the Post HCP Era
Abstract
Diffusion imaging is a critical component in the pursuit of developing a better understanding of the human brain. Recent technical advances promise enabling the advancement in the quality of data that can be obtained. In this review the context for different approaches relative to the Human Connectome Project are compared. Significant new gains are anticipated from the use of high-performance head gradients. These gains can be particularly large when the high-performance gradients are employed together with ultrahigh magnetic fields. Transmit array designs are critical in realizing high accelerations in diffusion-weighted (d)MRI acquisitions, while maintaining large field of view (FOV) coverage, and several techniques for optimal signal-encoding are now available. Reconstruction and processing pipelines that precisely disentangle the acquired neuroanatomical information are established and provide the foundation for the application of deep learning in the advancement of dMRI for complex tissues. Level of Evidence: 3 Technical Efficacy Stage: Stage 3.
Keywords: diffusion imaging; human connectome project; ultra high field.
© 2020 International Society for Magnetic Resonance in Medicine.
Figures

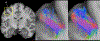



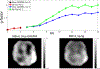

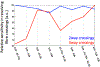



References
-
- Van Essen DC, Ugurbil K, Auerbach E, Barch D, Behrens TE, Bucholz R, Chang A, Chen L, Corbetta M, Curtiss SW, Della Penna S, Feinberg D, Glasser MF, Harel N, Heath AC, Larson-Prior L, Marcus D, Michalareas G, Moeller S, Oostenveld R, Petersen SE, Prior F, Schlaggar BL, Smith SM, Snyder AZ, Xu J, Yacoub E, Consortium WU-MH. The Human Connectome Project: a data acquisition perspective. Neuroimage 2012;62(4):2222–2231. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

