Crosstalk between Inflammation and the BBB in Stroke
- PMID: 32562523
- PMCID: PMC7770647
- DOI: 10.2174/1570159X18666200620230321
Crosstalk between Inflammation and the BBB in Stroke
Abstract
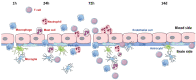
The blood-brain barrier (BBB), which is located at the interface between the central nervous system (CNS) and the circulatory system, is instrumental in establishing and maintaining the microenvironmental homeostasis of the CNS. BBB disruption following stroke promotes inflammation by enabling leukocytes, T cells and other immune cells to migrate via both the paracellular and transcellular routes across the BBB and to infiltrate the CNS parenchyma. Leukocytes promote the removal of necrotic tissues and neuronal recovery, but they also aggravate BBB injury and exacerbate stroke outcomes, especially after late reperfusion. Moreover, the swelling of astrocyte endfeet is thought to contribute to the 'no-reflow' phenomenon observed after cerebral ischemia, that is, blood flow cannot return to capillaries after recanalization of large blood vessels. Pericyte recruitment and subsequent coverage of endothelial cells (ECs) alleviate BBB disruption, which causes the transmigration of inflammatory cells across the BBB to be a dynamic process. Furthermore, interneurons and perivascular microglia also make contacts with ECs, astrocytes and pericytes to establish the neurovascular unit. BBB-derived factors after cerebral ischemia triggered microglial activation. During the later stage of injury, microglia remain associated with brain ECs and contribute to repair mechanisms, including postinjury angiogenesis, by acquiring a protective phenotype, which possibly occurs through the release of microglia-derived soluble factors. Taken together, we reviewed dynamic and bidirectional crosstalk between inflammation and the BBB during stroke and revealed targeted interventions based on the crosstalk between inflammation and the BBB, which will provide novel insights for developing new therapeutic strategies.
Keywords: Stroke; adaptive immunity; blood-brain barrier; inflammation; innate immunity; treatment.
Copyright© Bentham Science Publishers; For any queries, please email at epub@benthamscience.net.
Figures
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
