Posterior Oropharyngeal Saliva for the Detection of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)
- PMID: 32562544
- PMCID: PMC7337706
- DOI: 10.1093/cid/ciaa797
Posterior Oropharyngeal Saliva for the Detection of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)
Abstract
Background: The coronavirus disease 2019 (COVID-19) pandemic has put tremendous pressure on the healthcare system worldwide. Diagnostic testing remained one of the limiting factors for early identification and isolation of infected patients. This study aimed to evaluate posterior oropharyngeal saliva (POPS) for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) detection among patients with confirmed or suspected COVID-19.
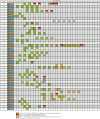
Methods: The laboratory information system was searched retrospectively for all respiratory specimens and POPS requested for SARS-CoV-2 RNA detection between 1 February 2020 and 15 April 2020. The agreement and diagnostic performance of POPS against NPsp were evaluated.
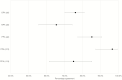
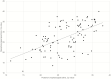
Results: A total of 13772 specimens were identified during the study period, including 2130 POPS and 8438 nasopharyngeal specimens (NPsp). Two hundred and twenty-nine same-day POPS-NPsp paired were identified with POPS and NPsp positivity of 61.5% (95% confidence interval [CI] 55.1-67.6%) and 53.3% (95% CI 46.8-59.6%). The overall, negative and positive percent agreement were 76.0% (95% CI 70.2-80.9%), 65.4% (95% CI 55.5-74.2%), 85.2% (95% CI 77.4-90.8%). Better positive percent agreement was observed in POPS-NPsp obtained within 7 days (96.6%, 95% CI 87.3-99.4%) compared with after 7 days of symptom onset (75.0%, 95% CI 61.4-85.2%). Among the 104 positive pairs, the mean difference in Cp value was 0.26 (range: 12.63 to -14.74), with an overall higher Cp value in NPsp (Pearson coefficient 0.579). No significant temporal variation was noted between the 2 specimen types.
Conclusions: POPS is an acceptable alternative specimen to nasopharyngeal specimen for the detection of SARS-CoV-2.
Keywords: COVID-19; mass screening; pandemic; saliva; severe acute respiratory syndrome coronavirus 2.
© The Author(s) 2020. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures
Comment in
-
Posterior Oropharyngeal Saliva for the Detection of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2).Clin Infect Dis. 2021 Aug 2;73(3):555-557. doi: 10.1093/cid/ciaa1181. Clin Infect Dis. 2021. PMID: 32770241 Free PMC article. No abstract available.
References
-
- World Health Organization. Laboratory testing for coronavirus disease 2019 (COVID-19) in suspected human cases: interim guidance, 2 March 2020. World Health Organization.2020. Available at: https://apps.who.int/iris/handle/10665/331329. Accessed 6 May 2020.
-
- Centers for Disease Control and Prevention. Interim guidelines for collecting, handling, and testing clinical specimens from persons for coronavirus disease 2019 (covid-19), 14 April 2020 2020. Available at: https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specim.... Accessed 27 April 2020.
-
- Mufson S, Timberg C, Tiku N. When these Boston doctors ran out of virus-testing swabs, they mobilized an army of 3-D printers. The Washington Post.2020. Available at: https://www.washingtonpost.com/climate-environment/2020/04/22/nasal-swab.... Accessed 27 April 2020.
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

