Cellular processes driving gastrulation in the avian embryo
- PMID: 32562871
- PMCID: PMC7511600
- DOI: 10.1016/j.mod.2020.103624
Cellular processes driving gastrulation in the avian embryo
Abstract
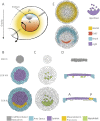
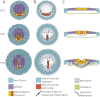
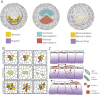
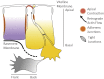
Gastrulation consists in the dramatic reorganisation of the epiblast, a one-cell thick epithelial sheet, into a multilayered embryo. In chick, the formation of the internal layers requires the generation of a macroscopic convection-like flow, which involves up to 50,000 epithelial cells in the epiblast. These cell movements locate the mesendoderm precursors into the midline of the epiblast to form the primitive streak. There they acquire a mesenchymal phenotype, ingress into the embryo and migrate outward to populate the inner embryonic layers. This review covers what is currently understood about how cell behaviours ultimately cause these morphogenetic events and how they are regulated. We discuss 1) how the biochemical patterning of the embryo before gastrulation creates compartments of differential cell behaviours, 2) how the global epithelial flows arise from the coordinated actions of individual cells, 3) how the cells delaminate individually from the epiblast during the ingression, and 4) how cells move after the ingression following stereotypical migration routes. We conclude by exploring new technical advances that will facilitate future research in the chick model system.
Keywords: Cell flows; Chick embryo; Gastrulation; Intercalation; Morphogenesis; Patterning.
Copyright © 2020 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors have no competing interests to declare.
Figures
References
-
- Acloque H., Ocana O.H., Abad D., Stern C.D., Nieto M.A. Snail2 and Zeb2 repress P-cadherin to define embryonic territories in the chick embryo. Development. 2017;144:649–656. - PubMed
-
- Alev C., Wu Y.P., Nakaya Y., Sheng G.J. Decoupling of amniote gastrulation and streak formation reveals a morphogenetic unity in vertebrate mesoderm induction. Development. 2013;140:2691–2696. - PubMed
-
- Arendt D., Nubler-Jung K. Rearranging gastrulation in the name of yolk: evolution of gastrulation in yolk-rich amniote eggs. Mech. Dev. 1999;81:3–22. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

