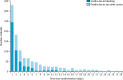
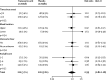
Effects of a high-dose 24-h infusion of tranexamic acid on death and thromboembolic events in patients with acute gastrointestinal bleeding (HALT-IT): an international randomised, double-blind, placebo-controlled trial
- PMID: 32563378
- PMCID: PMC7306161
- DOI: 10.1016/S0140-6736(20)30848-5
Effects of a high-dose 24-h infusion of tranexamic acid on death and thromboembolic events in patients with acute gastrointestinal bleeding (HALT-IT): an international randomised, double-blind, placebo-controlled trial
Abstract
Background: Tranexamic acid reduces surgical bleeding and reduces death due to bleeding in patients with trauma. Meta-analyses of small trials show that tranexamic acid might decrease deaths from gastrointestinal bleeding. We aimed to assess the effects of tranexamic acid in patients with gastrointestinal bleeding.
Methods: We did an international, multicentre, randomised, placebo-controlled trial in 164 hospitals in 15 countries. Patients were enrolled if the responsible clinician was uncertain whether to use tranexamic acid, were aged above the minimum age considered an adult in their country (either aged 16 years and older or aged 18 years and older), and had significant (defined as at risk of bleeding to death) upper or lower gastrointestinal bleeding. Patients were randomly assigned by selection of a numbered treatment pack from a box containing eight packs that were identical apart from the pack number. Patients received either a loading dose of 1 g tranexamic acid, which was added to 100 mL infusion bag of 0·9% sodium chloride and infused by slow intravenous injection over 10 min, followed by a maintenance dose of 3 g tranexamic acid added to 1 L of any isotonic intravenous solution and infused at 125 mg/h for 24 h, or placebo (sodium chloride 0·9%). Patients, caregivers, and those assessing outcomes were masked to allocation. The primary outcome was death due to bleeding within 5 days of randomisation; analysis excluded patients who received neither dose of the allocated treatment and those for whom outcome data on death were unavailable. This trial was registered with Current Controlled Trials, ISRCTN11225767, and ClinicalTrials.gov, NCT01658124.
Findings: Between July 4, 2013, and June 21, 2019, we randomly allocated 12 009 patients to receive tranexamic acid (5994, 49·9%) or matching placebo (6015, 50·1%), of whom 11 952 (99·5%) received the first dose of the allocated treatment. Death due to bleeding within 5 days of randomisation occurred in 222 (4%) of 5956 patients in the tranexamic acid group and in 226 (4%) of 5981 patients in the placebo group (risk ratio [RR] 0·99, 95% CI 0·82-1·18). Arterial thromboembolic events (myocardial infarction or stroke) were similar in the tranexamic acid group and placebo group (42 [0·7%] of 5952 vs 46 [0·8%] of 5977; 0·92; 0·60 to 1·39). Venous thromboembolic events (deep vein thrombosis or pulmonary embolism) were higher in tranexamic acid group than in the placebo group (48 [0·8%] of 5952 vs 26 [0·4%] of 5977; RR 1·85; 95% CI 1·15 to 2·98).
Interpretation: We found that tranexamic acid did not reduce death from gastrointestinal bleeding. On the basis of our results, tranexamic acid should not be used for the treatment of gastrointestinal bleeding outside the context of a randomised trial.
Funding: UK National Institute for Health Research Health Technology Assessment Programme.
Copyright © 2020 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Figures
Comment in
-
Tranexamic acid for severe gastrointestinal bleeding.Lancet. 2020 Jun 20;395(10241):1885-1886. doi: 10.1016/S0140-6736(20)30975-2. Lancet. 2020. PMID: 32563356 No abstract available.
-
In adults with severe acute GI bleeding, tranexamic acid did not reduce death due to bleeding at 5 days.Ann Intern Med. 2020 Oct 20;173(8):JC46. doi: 10.7326/ACPJ202010200-046. Ann Intern Med. 2020. PMID: 33075257
-
Does tranexamic acid reduce 5-day death due to bleeding in acute gastrointestinal bleed?CJEM. 2021 Mar;23(2):164-165. doi: 10.1007/s43678-020-00053-z. Epub 2021 Jan 4. CJEM. 2021. PMID: 33709351 No abstract available.
-
[Focus general intensive care medicine. Intensive care studies from 2020/2021].Anaesthesist. 2021 Oct;70(10):888-894. doi: 10.1007/s00101-021-00976-x. Epub 2021 Jul 29. Anaesthesist. 2021. PMID: 34324037 Free PMC article. German. No abstract available.
References
-
- van Leerdam ME. Epidemiology of acute upper gastrointestinal bleeding. Best Pract Res Clin Gastroenterol. 2008;22:209–224. - PubMed
-
- Hearnshaw SA, Logan RF, Lowe D, Travis SP, Murphy MF, Palmer KR. Acute upper gastrointestinal bleeding in the UK: patient characteristics, diagnoses and outcomes in the 2007 UK audit. Gut. 2011;60:1327–1335. - PubMed
-
- Oakland K, Guy R, Uberoi R. Acute lower GI bleeding in the UK: patient characteristics, interventions and outcomes in the first nationwide audit. Gut. 2018;67:654–662. - PubMed
-
- Myles PS, Smith JA, Forbes A. Tranexamic acid in patients undergoing coronary-artery surgery. N Engl J Med. 2017;376:136–148. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical