A promising antiviral candidate drug for the COVID-19 pandemic: A mini-review of remdesivir
- PMID: 32563812
- PMCID: PMC7834743
- DOI: 10.1016/j.ejmech.2020.112527
A promising antiviral candidate drug for the COVID-19 pandemic: A mini-review of remdesivir
Abstract
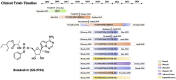
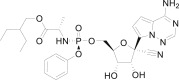
Remdesivir (GS-5734), a viral RNA-dependent RNA polymerase (RdRP) inhibitor that can be used to treat a variety of RNA virus infections, is expected to be an effective treatment for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. On May 1, 2020, The U.S. Food and Drug Administration (FDA) has granted Emergency Use Authorization (EUA) for remdesivir to treat COVID-19 patients. In light of the COVID-19 pandemic, this review presents comprehensive information on remdesivir, including information regarding the milestones, intellectual properties, anti-coronavirus mechanisms, preclinical research and clinical trials, and in particular, the chemical synthesis, pharmacology, toxicology, pharmacodynamics and pharmacokinetics of remdesivir. Furthermore, perspectives regarding the use of remdesivir for the treatment of COVID-19 are also discussed.
Keywords: Antiviral; COVID-19; Coronavirus; RdRP inhibitor; Remdesivir (GS-5734).
Copyright © 2020 Elsevier Masson SAS. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declared that they have no conflicts of interest to this work.
Figures


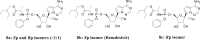


References
-
- Li G., De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV) Nat. Rev. Drug Discov. 2020;19:149–150. - PubMed
-
- Hui D.S., Azhar E.I., Madani T.A., Ntoumi F., Kock R., Dar O., Ippolito G., McHugh T.D., Memish Z.A., Drosten C., Zumla A., Petersen E. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health - the latest 2019 novel coronavirus outbreak in Wuhan, China. Int. J. Infect. Dis. 2020;91:264–266. - PMC - PubMed
-
- Ghinai I., McPherson T.D., Hunter J.C., Kirking H.L., Christiansen D., Joshi K., Rubin R., Morales-Estrada S., Black S.R., Pacilli M., Fricchione M.J., Chugh R.K., Walblay K.A., Ahmed N.S., Stoecker W.C., Hasan N.F., Burdsall D.P., Reese H.E., Wallace M., Wang C., Moeller D., Korpics J., Novosad S.A., Benowitz I., Jacobs M.W., Dasari V.S., Patel M.T., Kauerauf J., Charles E.M., Ezike N.O., Chu V., Midgley C.M., Rolfes M.A., Gerber S.I., Lu X., Lindstrom S., Verani J.R., Layden J.E., Illinois C.-I. First known person-to-person transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the USA. Lancet. 2020;395:1137–1144. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

