Transient Intermittent Hyperglycemia Accelerates Atherosclerosis by Promoting Myelopoiesis
- PMID: 32564710
- PMCID: PMC7486277
- DOI: 10.1161/CIRCRESAHA.120.316653
Transient Intermittent Hyperglycemia Accelerates Atherosclerosis by Promoting Myelopoiesis
Abstract
Rationale: Treatment efficacy for diabetes mellitus is largely determined by assessment of HbA1c (glycated hemoglobin A1c) levels, which poorly reflects direct glucose variation. People with prediabetes and diabetes mellitus spend >50% of their time outside the optimal glucose range. These glucose variations, termed transient intermittent hyperglycemia (TIH), appear to be an independent risk factor for cardiovascular disease, but the pathological basis for this association is unclear.
Objective: To determine whether TIH per se promotes myelopoiesis to produce more monocytes and consequently adversely affects atherosclerosis.
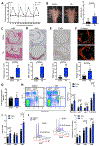
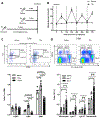
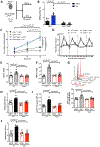
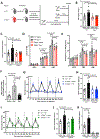
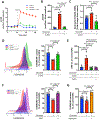
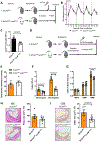
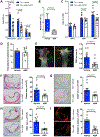
Methods and results: To create a mouse model of TIH, we administered 4 bolus doses of glucose at 2-hour intervals intraperitoneally once to WT (wild type) or once weekly to atherosclerotic prone mice. TIH accelerated atherogenesis without an increase in plasma cholesterol, seen in traditional models of diabetes mellitus. TIH promoted myelopoiesis in the bone marrow, resulting in increased circulating monocytes, particularly the inflammatory Ly6-Chi subset, and neutrophils. Hematopoietic-restricted deletion of S100a9, S100a8, or its cognate receptor Rage prevented monocytosis. Mechanistically, glucose uptake via GLUT (glucose transporter)-1 and enhanced glycolysis in neutrophils promoted the production of S100A8/A9. Myeloid-restricted deletion of Slc2a1 (GLUT-1) or pharmacological inhibition of S100A8/A9 reduced TIH-induced myelopoiesis and atherosclerosis.
Conclusions: Together, these data provide a mechanism as to how TIH, prevalent in people with impaired glucose metabolism, contributes to cardiovascular disease. These findings provide a rationale for continual glucose control in these patients and may also suggest that strategies aimed at targeting the S100A8/A9-RAGE (receptor for advanced glycation end products) axis could represent a viable approach to protect the vulnerable blood vessels in diabetes mellitus. Graphic Abstract: A graphic abstract is available for this article.
Keywords: atherosclerosis; diabetes mellitus; inflammation; metabolism; stem cells.
Conflict of interest statement
DISCLOSURES
No competing interests
Figures
Comment in
-
Transient Intermittent Hyperglycemia-Enhanced Myelopoiesis and Atherosclerosis: Short and Sweet.Circ Res. 2020 Sep 11;127(7):893-895. doi: 10.1161/CIRCRESAHA.120.317797. Epub 2020 Sep 10. Circ Res. 2020. PMID: 32910740 No abstract available.
References
-
- Beckman JA, Creager MA and Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. Jama. 2002;287:2570–81. - PubMed
-
- Nagareddy PR, Murphy AJ, Stirzaker RA, Hu Y, Yu S, Miller RG, Ramkhelawon B, Distel E, Westerterp M, Huang LS, Schmidt AM, Orchard TJ, Fisher EA, Tall AR and Goldberg IJ. Hyperglycemia promotes myelopoiesis and impairs the resolution of atherosclerosis. Cell metabolism. 2013;17:695–708. - PMC - PubMed
-
- Boussageon R, Bejan-Angoulvant T, Saadatian-Elahi M, Lafont S, Bergeonneau C, Kassai B, Erpeldinger S, Wright JM, Gueyffier F and Cornu C. Effect of intensive glucose lowering treatment on all cause mortality, cardiovascular death, and microvascular events in type 2 diabetes: meta-analysis of randomised controlled trials. BMJ. 2011;343:d4169. - PMC - PubMed
-
- Monnier L, Colette C, Dejager S and Owens D. Residual dysglycemia when at target HbA(1c) of 7% (53mmol/mol) in persons with type 2 diabetes. Diabetes Res Clin Pract. 2014;104:370–5. - PubMed
-
- Monnier L and Colette C. Postprandial and basal hyperglycaemia in type 2 diabetes: Contributions to overall glucose exposure and diabetic complications. Diabetes Metab. 2015;41:6S9–6S15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

