A novel method for calculating beta band burst durations in Parkinson's disease using a physiological baseline
- PMID: 32565222
- PMCID: PMC7437353
- DOI: 10.1016/j.jneumeth.2020.108811
A novel method for calculating beta band burst durations in Parkinson's disease using a physiological baseline
Abstract
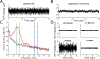
Background: Pathologically prolonged bursts of neural activity in the 8-30 Hz frequency range in Parkinson's disease have been measured using high power event detector thresholds.
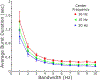
New method: This study introduces a novel method for determining beta bursts using a power baseline based on spectral activity that overlapped a simulated 1/f spectrum. We used resting state local field potentials from people with Parkinson's disease and a simulated 1/f signal to measure beta burst durations, to demonstrate how tuning parameters (i.e., bandwidth and center frequency) affect burst durations, to compare burst duration distributions with high power threshold methods, and to study the effect of increasing neurostimulation intensities on burst duration.
Results: The baseline method captured a broad distribution of resting state beta band burst durations. Mean beta band burst durations were significantly shorter on compared to off neurostimulation (p = 0.0046), and their distribution shifted towards that of the 1/f spectrum during increasing intensities of stimulation.
Comparison with existing methods: High power event detection methods, measure duration of higher power bursts and omit portions of the neural signal. The baseline method captured the broadest distribution of burst durations and was more sensitive than high power detection methods in demonstrating the effect of neurostimulation on beta burst duration.
Conclusions: The baseline method captured a broad range of fluctuations in beta band neural activity and demonstrated that subthalamic neurostimulation shortened burst durations in a dose (intensity) dependent manner, suggesting that beta burst duration is a useful control variable for closed loop algorithms.
Keywords: Beta fluctuations; Burst durations; Deep brain stimulation; Local field potentials; Parkinson’s disease; Thresholding.
Copyright © 2020 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest H.B.S. is a member of the Medtronic Inc. Clinical Advisory Board.
Figures



References
-
- Afzal MF, Velisar A, Anidi C, Neuville R, Prabhakar V, Bronte-Stewart H, 2019. Proceedings #61: Subthalamic Neural Closed-loop Deep Brain Stimulation for Bradykinesia in Parkinson’s Disease. Brain Stimulation 12, e152–e154. 10.1016/j.brs.2019.03.019 - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

