Early Pandemic Evaluation and Enhanced Surveillance of COVID-19 (EAVE II): protocol for an observational study using linked Scottish national data
- PMID: 32565483
- PMCID: PMC7311023
- DOI: 10.1136/bmjopen-2020-039097
Early Pandemic Evaluation and Enhanced Surveillance of COVID-19 (EAVE II): protocol for an observational study using linked Scottish national data
Abstract
Introduction: Following the emergence of the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in December 2019 and the ensuing COVID-19 pandemic, population-level surveillance and rapid assessment of the effectiveness of existing or new therapeutic or preventive interventions are required to ensure that interventions are targeted to those at highest risk of serious illness or death from COVID-19. We aim to repurpose and expand an existing pandemic reporting platform to determine the attack rate of SARS-CoV-2, the uptake and effectiveness of any new pandemic vaccine (once available) and any protective effect conferred by existing or new antimicrobial drugs and other therapies.
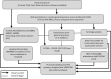
Methods and analysis: A prospective observational cohort will be used to monitor daily/weekly the progress of the COVID-19 epidemic and to evaluate the effectiveness of therapeutic interventions in approximately 5.4 million individuals registered in general practices across Scotland. A national linked dataset of patient-level primary care data, out-of-hours, hospitalisation, mortality and laboratory data will be assembled. The primary outcomes will measure association between: (A) laboratory confirmed SARS-CoV-2 infection, morbidity and mortality, and demographic, socioeconomic and clinical population characteristics; and (B) healthcare burden of COVID-19 and demographic, socioeconomic and clinical population characteristics. The secondary outcomes will estimate: (A) the uptake (for vaccines only); (B) effectiveness; and (C) safety of new or existing therapies, vaccines and antimicrobials against SARS-CoV-2 infection. The association between population characteristics and primary outcomes will be assessed via multivariate logistic regression models. The effectiveness of therapies, vaccines and antimicrobials will be assessed from time-dependent Cox models or Poisson regression models. Self-controlled study designs will be explored to estimate the risk of therapeutic and prophylactic-related adverse events.
Ethics and dissemination: We obtained approval from the National Research Ethics Service Committee, Southeast Scotland 02. The study findings will be presented at international conferences and published in peer-reviewed journals.
Keywords: epidemiology; public health; respiratory medicine (see thoracic medicine).
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Conflicts of Interest: CRS reports grants from the UK National Institute for Health Research, Medical Research Council and New Zealand Health Research Council, and The Ministry of Business, Innovation and Employment during the conduct of (and related to) the study. CR reports grants from the UK Medical Research Council, CSO during the conduct of (and related to) the study. CR is a member of the Scottish Government’s Chief Medical Officer’s COVID-19 Advisory Group. He is also a member of the UK SPI-M committee and the Commission Human Medicines COVID-19 Vaccine Safety Working Group. The views represented in this article do not represent the views of the UK or Scottish Government. JM is Incident Director for COVID-19 at Public Health Scotland and reports no conflicts of interest. LDR serves on a number of Scottish Government Advisory Groups, including COVID-19. MW is a member of the SPI-M advisory committee for the UK Government and the Covid-19 Advisory Group for the Scottish Government. DK is a director of Albasoft Ltd and a health informatician providing technical advice and support to the research community. HRS reports grants from the UK Medical Research Council during the conduct of the study. AS is a member of the Scottish Government’s Chief Medical Officer’s COVID-19 Advisory Group. The views represented in this article do not represent the views of the Scottish Government. EV, RG, LM, DM and JM report no conflicts of interest.
Figures
Comment in
-
Characterising adults in Scotland who are not vaccinated against COVID-19.Lancet. 2022 Sep 24;400(10357):993-995. doi: 10.1016/S0140-6736(22)01653-1. Lancet. 2022. PMID: 36154687 Free PMC article. No abstract available.
References
-
- Centers for Disease Control and Prevention Inlfuenza (flu). pandemic influenza. past pandemics. Available: https://www.cdc.gov/flu/pandemic-resources/basics/past-pandemics.html [Accessed 23 Mar 2020].
-
- World Health Organization Influenza. past pandemics. Available: http://www.euro.who.int/en/health-topics/communicable-diseases/influenza... [Accessed 23 Mar 2020].
-
- LeDuc JW, Barry MA. Sars: the first pandemic of the 21st century. Emerg Infect Dis 2004;10:e26. 10.3201/eid1011.040797_02 - DOI
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
