Methods for Hydroxamic Acid Synthesis
- PMID: 32565717
- PMCID: PMC7304568
- DOI: 10.2174/1385272823666190424142821
Methods for Hydroxamic Acid Synthesis
Abstract
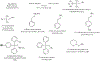
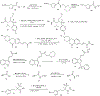
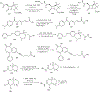
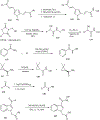
Substituted hydroxamic acid is one of the most extensively studied pharmacophores because of their ability to chelate biologically important metal ions to modulate various enzymes, such as HDACs, urease, metallopeptidase, and carbonic anhydrase. Syntheses and biological studies of various classes of hydroxamic acid derivatives have been reported in numerous research articles in recent years but this is the first review article dedicated to their synthetic methods and their application for the synthesis of these novel molecules. In this review article, commercially available reagents and preparation of hydroxylamine donating reagents have also been described.
Keywords: Hydroxamic acids; catalytic reaction; coupling reactions; direct synthesis; hydroxy lamine; mutagens.
Conflict of interest statement
CONFLICT OF INTEREST The author declares no conflict of interest, financial or otherwise.
Figures

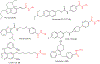



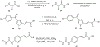
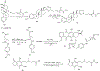


References
-
- Neff C; Bellot F; Waern JB; Lambert F; Brandei J; Serratrice G; Gaboriau F; Policar C, Glycosiderophores: Synthesis of tris-hydroxamate siderophores based on a galactose or glycero central scaffold, Fe(III) complexation studies. J. Inorg. Biochem, 2012,112, 59–67. - PubMed
-
- Codd R, Traversing the coordination chemistry and chemical biology of hydroxamic acids. Coord. Chem. Rev, 2008, 252, 1387–1408.
-
- Griffith DM; Szocs B; Keogh T; Suponitsky KY; Farkas E; Buglyo P; Marmion CJ, Suberoylanilide hydroxamic acid, a potent histone deacetylase inhibitor; its X-ray crystal structure and solid state and solution studies of its Zn(II), Ni(II), Cu(II) and Fe(III) complexes. J. Inorg. Biochem, 2011,705,763–9. - PubMed
-
- Locock KES; Yamamoto I; Tran P; Hanrahan JR; Chebib M; Johnston GAR; Allan RD, γ-Aminobutyric Acid(C) (GABAC) Selective antagonists derived from the bioisosteric modification of 4-aminocyclopent-1-enecarboxylic acid: Amides and Hydroxamates. J. Med. Chem, 2013, 56, 5626–5630. - PubMed
-
- Flipo M; Charton J; Hocine A; Dassonneville S; Deprez B; Deprez-Poulain R Hydroxamates: relationships between structure and plasma stability. J. Med. Chem, 2009, 52, 6790–802. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
