hnRNPA2/B1 Ameliorates LPS-Induced Endothelial Injury through NF- κ B Pathway and VE-Cadherin/ β-Catenin Signaling Modulation In Vitro
- PMID: 32565727
- PMCID: PMC7277030
- DOI: 10.1155/2020/6458791
hnRNPA2/B1 Ameliorates LPS-Induced Endothelial Injury through NF- κ B Pathway and VE-Cadherin/ β-Catenin Signaling Modulation In Vitro
Abstract
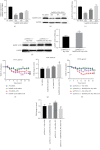
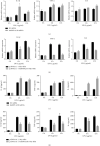
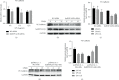
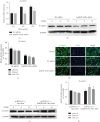
Heterogeneous nuclear ribonucleoprotein A2/B1 (hnRNPA2/B1) is a protein involved in the regulation of RNA processing, cell metabolism, migration, proliferation, and apoptosis. However, the effect of hnRNPA2/B1 on injured endothelial cells (ECs) remains unclear. We investigated the effect of hnRNPA2/B1 on lipopolysaccharide- (LPS-) induced vascular endothelial injury in human umbilical vein endothelial cells (HUVECs) and the underlying mechanisms. LPS was used to induce EC injury, and the roles of hnRNPA2/B1 in EC barrier dysfunction and inflammatory responses were measured by testing endothelial permeability and the expression of inflammatory factors after the suppression and overexpression of hnRNPA2/B1. To explore the underlying mechanism by which hnRNPA2/B1 regulates endothelial injury, we studied the VE-cadherin/β-catenin pathway and NF-κB activation in HUVECs. The results showed that hnRNPA2/B1 was elevated in LPS-stimulated HUVECs. Moreover, knockdown of hnRNPA2/B1 aggravated endothelial injury by increasing EC permeability and promoting the secretion of the inflammatory cytokines TNF-α, IL-1β, and IL-6. Overexpression of hnRNPA2/B1 can reduce the permeability and inflammatory response of HUVEC stimulated by LPS in vitro, while increasing the expression of VE-Cadherin and β-catenin. Furthermore, the suppression of hnRNPA2/B1 increased the LPS-induced NF-κB activation and reduced the VE-cadherin/β-catenin pathway. Taken together, these results suggest that hnRNPA2/B1 can regulate LPS-induced EC damage through regulating the NF-κB and VE-cadherin/β-catenin pathways.
Copyright © 2020 Yi Chen et al.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

