Progranulin Improves Acute Lung Injury through Regulating the Differentiation of Regulatory T Cells and Interleukin-10 Immunomodulation to Promote Macrophage Polarization
- PMID: 32565732
- PMCID: PMC7281846
- DOI: 10.1155/2020/9704327
Progranulin Improves Acute Lung Injury through Regulating the Differentiation of Regulatory T Cells and Interleukin-10 Immunomodulation to Promote Macrophage Polarization
Abstract
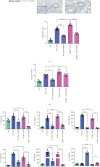
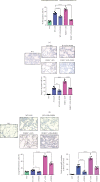
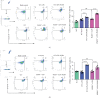
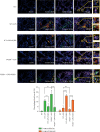
Progranulin (PGRN), which plays an anti-inflammatory role in acute lung injury (ALI), is promising as a potential drug. Studies have shown that regulatory T cells (Tregs) and interleukin- (IL-) 10 can repress inflammation and alleviate tissue damage during ALI. In this study, we built a lipopolysaccharide- (LPS-) induced ALI mouse model to illustrate the effect of PGRN on regulation of Treg differentiation and modulation of IL-10 promoting macrophage polarization. We found that the proportion of Tregs in splenic mononuclear cells and peripheral blood mononuclear cells was higher after treatment with PGRN. The increased proportion of Tregs after PGRN intratracheal instillation was consistent with the decreased severity of lung injury, the reduction of proinflammatory cytokines, and the increase of anti-inflammatory cytokines. In vitro, the percentages of CD4+CD25+FOXP3+ Tregs from splenic naïve CD4+ T cells increased after PGRN treatment. In further research, it was found that PGRN can regulate the anti-inflammatory factor IL-10 and affect the polarization of M1/M2 macrophages by upregulating IL-10. These findings show that PGRN likely plays a protective role in ALI by promoting Treg differentiation and activating IL-10 immunomodulation.
Copyright © 2020 Yan-qing Chen et al.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Regulatory T cell and macrophage crosstalk in acute lung injury: future perspectives.Cell Death Discov. 2023 Jan 16;9(1):9. doi: 10.1038/s41420-023-01310-7. Cell Death Discov. 2023. PMID: 36646692 Free PMC article. Review.
-
Curcumin regulates the differentiation of naïve CD4+T cells and activates IL-10 immune modulation against acute lung injury in mice.Biomed Pharmacother. 2020 May;125:109946. doi: 10.1016/j.biopha.2020.109946. Epub 2020 Jan 28. Biomed Pharmacother. 2020. PMID: 32004976
-
Luteolin Regulates the Differentiation of Regulatory T Cells and Activates IL-10-Dependent Macrophage Polarization against Acute Lung Injury.J Immunol Res. 2021 Jan 18;2021:8883962. doi: 10.1155/2021/8883962. eCollection 2021. J Immunol Res. 2021. PMID: 33532509 Free PMC article.
-
Recovery from acute lung injury can be regulated via modulation of regulatory T cells and Th17 cells.Scand J Immunol. 2018 Nov;88(5):e12715. doi: 10.1111/sji.12715. Epub 2018 Sep 27. Scand J Immunol. 2018. PMID: 30277586
-
Differential roles of regulatory T cells in acute respiratory infections.J Clin Invest. 2023 Jul 17;133(14):e170505. doi: 10.1172/JCI170505. J Clin Invest. 2023. PMID: 37463441 Free PMC article. Review.
Cited by
-
Serum Progranulin as a Potential Diagnostic Predictor in Neonatal Sepsis.Cureus. 2025 Jul 20;17(7):e88360. doi: 10.7759/cureus.88360. eCollection 2025 Jul. Cureus. 2025. PMID: 40687411 Free PMC article.
-
Regulatory T cell and macrophage crosstalk in acute lung injury: future perspectives.Cell Death Discov. 2023 Jan 16;9(1):9. doi: 10.1038/s41420-023-01310-7. Cell Death Discov. 2023. PMID: 36646692 Free PMC article. Review.
-
Molecular mechanisms and targeted therapy of progranulin in metabolic diseases.Front Endocrinol (Lausanne). 2025 Apr 11;16:1553794. doi: 10.3389/fendo.2025.1553794. eCollection 2025. Front Endocrinol (Lausanne). 2025. PMID: 40290306 Free PMC article. Review.
-
Impact of Lung Microbiota on COPD.Biomedicines. 2022 Jun 6;10(6):1337. doi: 10.3390/biomedicines10061337. Biomedicines. 2022. PMID: 35740358 Free PMC article. Review.
-
Contribution of αβ T cells to macrophage polarization and MSC recruitment and proliferation on titanium implants.Acta Biomater. 2023 Oct 1;169:605-624. doi: 10.1016/j.actbio.2023.07.052. Epub 2023 Aug 1. Acta Biomater. 2023. PMID: 37532133 Free PMC article.
References
-
- Force A. D. T., Ranieri V. M., Rubenfeld G. D., et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307(23):2526–2533. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

