[3 + 2] Cycloaddition with photogenerated azomethine ylides in β-cyclodextrin
- PMID: 32566032
- PMCID: PMC7296198
- DOI: 10.3762/bjoc.16.110
[3 + 2] Cycloaddition with photogenerated azomethine ylides in β-cyclodextrin
Abstract
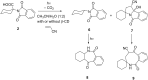
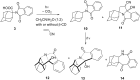
Stability constants for the inclusion complexes of cyclohexylphthalimide 2 and adamantylphthalimide 3 with β-cyclodextrin (β-CD) were determined by 1H NMR titration, K = 190 ± 50 M-1, and K = 2600 ± 600 M-1, respectively. Photochemical reactivity of the inclusion complexes 2@β-CD and 3@β-CD was investigated, and we found out that β-CD does not affect the decarboxylation efficiency, while it affects the subsequent photochemical H-abstraction, resulting in different product distribution upon irradiation in the presence of β-CD. The formation of ternary complexes with acrylonitrile (AN) and 2@β-CD or 3@β-CD was also essayed by 1H NMR. Although the formation of such complexes was suggested, stability constants could not be determined. Irradiation of 2@β-CD in the presence of AN in aqueous solution where cycloadduct 7 was formed highly suggests that decarboxylation and [3 + 2] cycloaddition take place in the ternary complex, whereas such a reactivity from bulky adamantane 3 is less likely. This proof of principle that decarboxylation and cycloaddition can be performed in the β-CD cavity has a significant importance for the design of new supramolecular systems for the control of photoreactivity.
Keywords: [3 + 2] cycloadditions; inclusion complexes; photochemistry; phthalimides; β-cyclodextrin.
Copyright © 2020, Sohora et al.; licensee Beilstein-Institut.
Figures

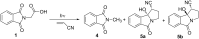


References
-
- Kobayashi S, Jørgensen K A, editors. Cycloaddition Reactions in Organic Synthesis. Weinheim: WILEY-VCH; 2002. - DOI
-
- Chiacchio U, Padwa A, Romeo G. Curr Org Chem. 2009;13:422–447. doi: 10.2174/138527209787582268. - DOI
-
- Nájera C, Sansano J M. Curr Org Chem. 2003;7:1105–1150. doi: 10.2174/1385272033486594. - DOI
LinkOut - more resources
Full Text Sources
