Strong-LAMP Assay Based on a Strongyloides spp.-Derived Partial Sequence in the 18S rRNA as Potential Biomarker for Strongyloidiasis Diagnosis in Human Urine Samples
- PMID: 32566039
- PMCID: PMC7281818
- DOI: 10.1155/2020/5265198
Strong-LAMP Assay Based on a Strongyloides spp.-Derived Partial Sequence in the 18S rRNA as Potential Biomarker for Strongyloidiasis Diagnosis in Human Urine Samples
Abstract
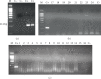
Human strongyloidiasis a soil-transmitted infection caused by Strongyloides stercoralis is one of the most neglected amongst the so-called Neglected Tropical Diseases (NTDs). S. stercoralis is a nematode, which is distributed worldwide; it has been estimated that it could affect millions of people, mainly in tropical and subtropical endemic regions. The difficulties of diagnosis lead to infection rates being underreported. Asymptomatic patients have chronic infections that can lead to severe hyperinfection syndrome or disseminated strongyloidiasis in immunocompromised patients. Strongyloidiasis can easily be misdiagnosed because conventional faecal-based techniques lack of sensitivity for the morphological identification of infective larvae in faeces. None of the currently used molecular methods have used urine samples as an alternative to faecal samples for diagnosing strongyloidiasis. This study was thus aimed at comparing, for the first time, the use of a new loop-mediated isothermal amplification (LAMP) molecular assay (Strong-LAMP) to traditional methods on patients' urine samples. Twenty-four urine samples were taken from patients included in a study involving two Spanish hospitals for strongyloidiasis screening using parasitological and serological tests. Strongyloides larvae were found in 11 patients' faecal samples, thereby ascertaining that they had the disease. Other patients had high antibody titres but no larvae were found in their faeces. All urine samples were analysed by PCR and Strong-LAMP assay. No amplification occurred when using PCR. Strong-LAMP led to detecting S. stercoralis DNA in urine samples from patients having previously confirmed strongyloidiasis by parasitological tests and/or a suspicion of being infected by serological ones. The Strong-LAMP assay is a useful molecular tool for research regarding strongyloidiasis in human urine samples. After further validation, the Strong-LAMP assay could also be used for complementary and effective diagnosis of strongyloidiasis in a clinical setting.
Copyright © 2020 Pedro Fernández-Soto et al.
Conflict of interest statement
The authors declare that there is no conflict of interest regarding the publication of this paper.
Figures



Similar articles
-
Strong-LAMP: A LAMP Assay for Strongyloides spp. Detection in Stool and Urine Samples. Towards the Diagnosis of Human Strongyloidiasis Starting from a Rodent Model.PLoS Negl Trop Dis. 2016 Jul 14;10(7):e0004836. doi: 10.1371/journal.pntd.0004836. eCollection 2016 Jul. PLoS Negl Trop Dis. 2016. PMID: 27415764 Free PMC article.
-
Application of real-time PCR for the detection of Strongyloides spp. in clinical samples in a reference center in Spain.Acta Trop. 2015 Feb;142:20-5. doi: 10.1016/j.actatropica.2014.10.020. Epub 2014 Nov 4. Acta Trop. 2015. PMID: 25447829
-
First field study using Strong-LAMP for diagnosis of strongyloidiasis in Cubal, Angola.Parasit Vectors. 2023 Oct 31;16(1):393. doi: 10.1186/s13071-023-06009-3. Parasit Vectors. 2023. PMID: 37907997 Free PMC article.
-
Molecular detection of Strongyloides stercoralis: Emerging factors and diagnostic utility.Clin Chim Acta. 2025 Mar 1;569:120184. doi: 10.1016/j.cca.2025.120184. Epub 2025 Feb 7. Clin Chim Acta. 2025. PMID: 39923908 Review.
-
Human infection with Strongyloides stercoralis and other related Strongyloides species.Parasitology. 2017 Mar;144(3):263-273. doi: 10.1017/S0031182016000834. Epub 2016 May 16. Parasitology. 2017. PMID: 27181117 Free PMC article. Review.
Cited by
-
Diagnosis of Human Endemic Mycoses Caused by Thermally Dimorphic Fungi: From Classical to Molecular Methods.J Fungi (Basel). 2024 Sep 6;10(9):637. doi: 10.3390/jof10090637. J Fungi (Basel). 2024. PMID: 39330397 Free PMC article. Review.
-
From past to present: opportunities and trends in the molecular detection and diagnosis of Strongyloides stercoralis.Parasit Vectors. 2023 Apr 11;16(1):123. doi: 10.1186/s13071-023-05763-8. Parasit Vectors. 2023. PMID: 37041645 Free PMC article. Review.
-
Loop-Mediated Isothermal Amplification as Point-of-Care Diagnosis for Neglected Parasitic Infections.Int J Mol Sci. 2020 Oct 28;21(21):7981. doi: 10.3390/ijms21217981. Int J Mol Sci. 2020. PMID: 33126446 Free PMC article. Review.
-
Concentration of Urine Samples Improves Sensitivity in Detection of Strongyloides-Specific IgG Antibody in Urine for Diagnosis of Strongyloidiasis.J Clin Microbiol. 2022 Jan 19;60(1):e0145421. doi: 10.1128/JCM.01454-21. Epub 2021 Oct 27. J Clin Microbiol. 2022. PMID: 34705534 Free PMC article.
-
Loop-Mediated Isothermal Amplification in Schistosomiasis.J Clin Med. 2021 Feb 1;10(3):511. doi: 10.3390/jcm10030511. J Clin Med. 2021. PMID: 33535489 Free PMC article. Review.
References
-
- Schad G. A., Aikens L. M., Smith G. Strongyloides stercoralis: is there a canonical migratory route through the host? The Journal of Parasitology. 1989;75(5):740–749. - PubMed
-
- Thanchomnang T., Intapan P. M., Sanpool O., et al. First molecular identification of Strongyloides fuelleborni in long-tailed macaques in Thailand and Lao People's Democratic Republic reveals considerable genetic diversity. Journal of Helminthology. 2019;93(5):608–615. doi: 10.1017/S0022149X18000512. - DOI - PubMed

