Zinc Oxide Nanoparticle Synergizes Sorafenib Anticancer Efficacy with Minimizing Its Cytotoxicity
- PMID: 32566073
- PMCID: PMC7275957
- DOI: 10.1155/2020/1362104
Zinc Oxide Nanoparticle Synergizes Sorafenib Anticancer Efficacy with Minimizing Its Cytotoxicity
Abstract
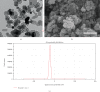
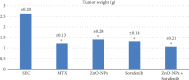
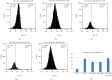
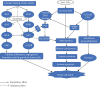
Cancer, as a group, represents the most important cause of death worldwide. Unfortunately, the available therapeutic approaches of cancer including surgery, chemotherapy, radiotherapy, and immunotherapy are unsatisfactory and represent a great challenge as many patients have cancer recurrence and severe side effects. Methotrexate (MTX) is a well-established (antineoplastic or cytotoxic) chemotherapy and immunosuppressant drug used to treat different types of cancer, but its usage requires high doses causing severe side effects. Therefore, we need a novel drug with high antitumor efficacy in addition to safety. The aim of this study was the evaluation of the antitumor efficacy of zinc oxide nanoparticle (ZnO-NPs) and sorafenib alone or in combination on solid Ehrlich carcinoma (SEC) in mice. Sixty adult female Swiss-albino mice were divided equally into 6 groups as follows: control, SEC, MTX, ZnO-NPs, sorafenib, and ZnO-NPs+sorafenib; all treatments continued for 4 weeks. ZnO-NPs were characterized by TEM, zeta potential, and SEM mapping. Data showed that ZnO-NPs synergized with sorafenib as a combination therapy to execute more effective and safer anticancer activity compared to monotherapy as showed by a significant reduction (P < 0.001) in tumor weight, tumor cell viability, and cancer tissue glutathione amount as well as by significant increase (P < 0.001) in tumor growth inhibition rate, DNA fragmentation, reactive oxygen species generation, the release of cytochrome c, and expression of the apoptotic gene caspase-3 in the tumor tissues with minimal changes in the liver, renal, and hematological parameters. Therefore, we suggest that ZnO-NPs might be a safe candidate in combination with sorafenib as a more potent anticancer. The safety of this combined treatment may allow its use in clinical trials.
Copyright © 2020 Ahmed Nabil et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Similar articles
-
Zinc oxide nanoparticles selectively induce apoptosis in human cancer cells through reactive oxygen species.Int J Nanomedicine. 2012;7:845-57. doi: 10.2147/IJN.S29129. Epub 2012 Feb 21. Int J Nanomedicine. 2012. PMID: 22393286 Free PMC article.
-
Apoptosis and oxidative stress as relevant mechanisms of antitumor activity and genotoxicity of ZnO-NPs alone and in combination with N-acetyl cysteine in tumor-bearing mice.Int J Nanomedicine. 2019 May 27;14:3911-3928. doi: 10.2147/IJN.S204757. eCollection 2019. Int J Nanomedicine. 2019. PMID: 31213808 Free PMC article.
-
Zinc oxide nanoparticles induce apoptosis and autophagy in human ovarian cancer cells.Int J Nanomedicine. 2017 Sep 5;12:6521-6535. doi: 10.2147/IJN.S140071. eCollection 2017. Int J Nanomedicine. 2017. PMID: 28919752 Free PMC article.
-
Zinc oxide nanoparticles: Synthesis, antiseptic activity and toxicity mechanism.Adv Colloid Interface Sci. 2017 Nov;249:37-52. doi: 10.1016/j.cis.2017.07.033. Epub 2017 Aug 26. Adv Colloid Interface Sci. 2017. PMID: 28923702 Review.
-
Zinc oxide nanoparticles for therapeutic purposes in cancer medicine.J Mater Chem B. 2020 Jun 21;8(23):4973-4989. doi: 10.1039/d0tb00739k. Epub 2020 May 19. J Mater Chem B. 2020. PMID: 32427264 Review.
Cited by
-
Antifungal Nano-Therapy in Veterinary Medicine: Current Status and Future Prospects.J Fungi (Basel). 2021 Jun 22;7(7):494. doi: 10.3390/jof7070494. J Fungi (Basel). 2021. PMID: 34206304 Free PMC article. Review.
-
Sorafenib-Based Drug Delivery Systems: Applications and Perspectives.Polymers (Basel). 2023 Jun 9;15(12):2638. doi: 10.3390/polym15122638. Polymers (Basel). 2023. PMID: 37376284 Free PMC article. Review.
-
Ultrastructural analysis of zinc oxide nanospheres enhances anti-tumor efficacy against Hepatoma.Front Oncol. 2022 Oct 27;12:933750. doi: 10.3389/fonc.2022.933750. eCollection 2022. Front Oncol. 2022. PMID: 36457501 Free PMC article.
-
The Potential Safe Antifibrotic Effect of Stem Cell Conditioned Medium and Nilotinib Combined Therapy by Selective Elimination of Rat Activated HSCs.Biomed Res Int. 2021 Mar 28;2021:6678913. doi: 10.1155/2021/6678913. eCollection 2021. Biomed Res Int. 2021. PMID: 33855079 Free PMC article.
-
Emerging biomaterials for tumor immunotherapy.Biomater Res. 2023 May 16;27(1):47. doi: 10.1186/s40824-023-00369-8. Biomater Res. 2023. PMID: 37194085 Free PMC article. Review.
References
-
- Ozaslan M., Karagoz I. D., Kilic I. H., Guldur M. E. Ehrlich ascites carcinoma. African Journal of Biotechnology. 2011;10:2375–2378.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

