Huangbai Liniment Accelerated Wound Healing by Activating Nrf2 Signaling in Diabetes
- PMID: 32566084
- PMCID: PMC7271242
- DOI: 10.1155/2020/4951820
Huangbai Liniment Accelerated Wound Healing by Activating Nrf2 Signaling in Diabetes
Abstract
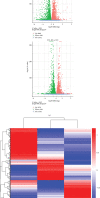
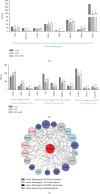
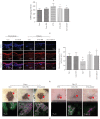
As a serious complication of diabetes, nonhealing skin ulcer leads to high mortality and disability in diabetic patients. However, limited therapy is available in managing diabetic wounds. In this study, RNA-seq technology was used to systematically investigate the effect of Huangbai (HB) liniment, a traditional Chinese medicine, on the streptozotocin- (STZ-) induced diabetic wound. HB liniment significantly accelerated the wound closure and enhanced the generation of extracellular matrix in diabetic rats, and oxidative stress was identified to play a vital role in HB-mediated wound healing. Importantly, HB liniment activated nuclear factor erythroid-derived 2-like 2 (Nrf2) and its downstream antioxidant genes (e.g., genes involved in glutathione system, thioredoxin system, and GAPDH generation as well as other antioxidant genes), which inhibited oxidative damage and apoptosis. By associating drug targets of HB liniment with Nrf2 and its downstream genes, 54 components in HB liniment were screened out, and the majority was from Cortex Phellodendri and Forsythia suspensa. Additionally, HB liniment enhanced TGF-β1 and reduced MMP9 level, accelerating wound healing in diabetes. The in vitro experiment showed HB facilitated cell proliferation and inhibited oxidative damage in high glucose-induced HaCaT cells. Our findings provided the experimental evidence for the treatment of diabetic wound with HB, clarified the potential mechanism of HB, and improved our understanding of diabetic wound healing.
Copyright © 2020 Jingjing Zhang et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures







References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

