Malaria vaccines since 2000: progress, priorities, products
- PMID: 32566259
- PMCID: PMC7283239
- DOI: 10.1038/s41541-020-0196-3
Malaria vaccines since 2000: progress, priorities, products
Abstract
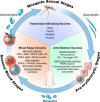
Malaria vaccine development entered a new era in 2015 when the pre-erythrocytic Plasmodium falciparum candidate RTS,S was favorably reviewed by the European Medicines Agency and subsequently introduced into national pilot implementation programs, marking the first human anti-parasite vaccine to pass regulatory scrutiny. Since the first trials published in 1997, RTS,S has been evaluated in a series of clinical trials culminating in Phase 3 testing, while testing of other pre-erythrocytic candidates (that target sporozoite- or liver-stage parasites), particularly whole sporozoite vaccines, has also increased. Interest in blood-stage candidates (that limit blood-stage parasite growth) subsided after disappointing human efficacy results, although new blood-stage targets and concepts may revive activity in this area. Over the past decade, testing of transmission-blocking vaccines (that kill mosquito/sexual-stage parasites) advanced to field trials and the first generation of placental malaria vaccines (that clear placenta-sequestering parasites) entered the clinic. Novel antigen discovery, human monoclonal antibodies, structural vaccinology, and improved platforms promise to expand on RTS,S and improve existing vaccine candidates.
Keywords: Malaria; Vaccines.
© This is a U.S. government work and not under copyright protection in the U.S.; foreign copyright protection may apply 2020.
Conflict of interest statement
Competing interestsThe authors declare no competing interests.
Figures
References
-
- WHO. (World Health Organization, Geneva, Switzerland, 2017).
-
- Clyde DF, Most H, McCarthy VC, Vanderberg JP. Immunization of man against sporozite-induced falciparum malaria. Am. J. Med. Sci. 1973;266:169–177. - PubMed
-
- Clyde DF. Immunization of man against falciparum and vivax malaria by use of attenuated sporozoites. Am. J. Trop. Med. Hyg. 1975;24:397–401. - PubMed
-
- Dame JB, et al. Structure of the gene encoding the immunodominant surface antigen on the sporozoite of the human malaria parasite Plasmodium falciparum. Science. 1984;225:593–599. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous