Applying lessons from the Ebola vaccine experience for SARS-CoV-2 and other epidemic pathogens
- PMID: 32566261
- PMCID: PMC7295741
- DOI: 10.1038/s41541-020-0204-7
Applying lessons from the Ebola vaccine experience for SARS-CoV-2 and other epidemic pathogens
Abstract
The world is experiencing an unprecedented global pandemic of coronavirus disease 2019 (COVID-19) caused by a novel coronavirus, Severe Acute Respiratory Syndrome-coronavirus-2 (SARS-CoV-2). Development of new vaccines and therapeutics are important to achieve long-term prevention and control of the virus. Experience gained in the development of vaccines for Ebola virus disease provide important lessons in the regulatory, clinical, and manufacturing process that can be applied to SARS-CoV-2 and other epidemic pathogens. This report outlines the main lessons learned by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA (MSD) during development of an Ebola Zaire vaccine (ERVEBO®) and looks ahead to critical lessons beyond vaccine development. It highlights focus areas for public-private partnership and regulatory harmonization that can be directly applied to current vaccine development efforts for SARS-CoV-2, while drawing attention to the need for parallel consideration of issues beyond development that are equally important to achieve global preparedness and response goals.
Keywords: Drug development; Drug regulation.
© The Author(s) 2020.
Conflict of interest statement
Competing interestsAll authors are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA (MSD) and may own stock or stock options in the company.
Figures
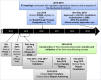

References
-
- World Health Organization. Ebola Situation Report—March 30, 2016. https://apps.who.int/ebola/current-situation/ebola-situation-report-30-m... (2020).
-
- Higgs E, et al. Accelerating vaccine development during the 2013-2016 West African Ebola virus disease outbreak. Curr. Top. Microbiol. Immunol. 2017;411:229–261. - PubMed
-
- International Federation of Pharmaceutical Manufacturers & Associations. The complex journey of a vaccine. https://www.ifpma.org/wp-content/uploads/2019/07/IFPMA-ComplexJourney-20... (2020).
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

