Detection of SARS-CoV-2 by bronchoscopy after negative nasopharyngeal testing: Stay vigilant for COVID-19
- PMID: 32566476
- PMCID: PMC7298516
- DOI: 10.1016/j.rmcr.2020.101120
Detection of SARS-CoV-2 by bronchoscopy after negative nasopharyngeal testing: Stay vigilant for COVID-19
Abstract
Purpose: Real-time polymerase chain reaction (RT-PCR) detection of severe acute respiratory syndrome coronavirus (SARS-CoV-2) is required for diagnosis of coronavirus disease 2019 (COVID-19). Sensitivity of RT-PCR nasopharyngeal (NP) testing is presumed to be high, but there is no gold standard against which this has been determined. The objective was to determine whether lower respiratory tract infection (LRTI), detected in bronchoalveolar lavage fluid (BALF), occurs in the absence of upper respiratory tract infection with clinical testing of both specimen types.
Methods: Between March 26, 2020 and April 17, 2020 at the University of Washington Medical Center all patients with BALF specimens clinically tested for SARS-CoV-2 were identified. We assessed the proportion of patients with positive RT-PCR for SARS-CoV-2 in BALF after negative NP testing. We describe 3 cases with positive testing in BALF.
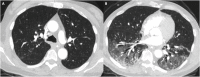
Results: Among 16 patients with BALF samples, 3 cases (19%) had SARS-CoV-2 detected in BALF. In Case 1, negative NP testing occurred early in the infection and respiratory symptoms may have been missed due to neurologic injury. In Case 2, outpatient diagnosis was aspiration pneumonia, but clinical suspicion remained high for COVID-19 at hospitalization based on epidemiological and clinical features. All 3 cases involved older adults (age >65 years), one of whom was immunosuppressed in the setting of lung transplantation (Case 3).
Conclusions: These data demonstrate that SARS-CoV-2 LRTI occurs in the presence of negative NP testing. NP testing may underestimate the prevalence of COVID-19 and has implications for spread of SARS-CoV2 in the community and healthcare setting.
© 2020 The Authors.
Conflict of interest statement
All authors report no significant financial conflicts of interest related to this manuscript. KJR receives funding from the 10.13039/100000002National Institutes of Health (K23HL138154-01) and the 10.13039/100000897Cystic Fibrosis Foundation (RAMOS17A0, LEASE16A3).
Figures
References
-
- Organization W.H. World Health Organization; 2020. Global Surveillance for COVID-19 Caused by Human Infection with COVID-19 Virus: Interim Guidance. 20 March 2020.
-
- Wölfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Müller M.A., Niemeyer D., Jones T.C., Vollmar P., Rothe C. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020:1–10. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous