Pathophysiology and Management of Type 2 Diabetes Mellitus Bone Fragility
- PMID: 32566682
- PMCID: PMC7262667
- DOI: 10.1155/2020/7608964
Pathophysiology and Management of Type 2 Diabetes Mellitus Bone Fragility
Abstract
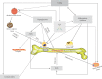
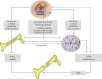
Individuals with type 2 diabetes mellitus (T2DM) have an increased risk of bone fragility fractures compared to nondiabetic subjects. This increased fracture risk may occur despite normal or even increased values of bone mineral density (BMD), and poor bone quality is suggested to contribute to skeletal fragility in this population. These concepts explain why the only evaluation of BMD could not be considered an adequate tool for evaluating the risk of fracture in the individual T2DM patient. Unfortunately, nowadays, the bone quality could not be reliably evaluated in the routine clinical practice. On the other hand, getting further insight on the pathogenesis of T2DM-related bone fragility could consent to ameliorate both the detection of the patients at risk for fracture and their appropriate treatment. The pathophysiological mechanisms underlying the increased risk of fragility fractures in a T2DM population are complex. Indeed, in T2DM, bone health is negatively affected by several factors, such as inflammatory cytokines, muscle-derived hormones, incretins, hydrogen sulfide (H2S) production and cortisol secretion, peripheral activation, and sensitivity. All these factors may alter bone formation and resorption, collagen formation, and bone marrow adiposity, ultimately leading to reduced bone strength. Additional factors such as hypoglycemia and the consequent increased propensity for falls and the direct effects on bone and mineral metabolism of certain antidiabetic medications may contribute to the increased fracture risk in this population. The purpose of this review is to summarize the literature evidence that faces the pathophysiological mechanisms underlying bone fragility in T2DM patients.
Copyright © 2020 C. Eller-Vainicher et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures